Can someone explain to me, in terms of the definition of vapor pressure, why a substance can only start to "boil" if vapor pressure = atmospheric pressure? Vapor pressure is the pressure exerted by a gas on its own liquid state, both atmospheric pressure/vapor pressure are pushing down on the liquid? I am just really confused by this.
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Vapor Pressure = Atmospheric Pressure --> Boiling...but why?
- Thread starter Rolling
- Start date
- Joined
- Jan 10, 2011
- Messages
- 476
- Reaction score
- 3
Vapor pressure is the gas pressure emitted by a liquid. It does not push down on the liquid. Instead, it is the pressure at which the gas and liquid are in equilibrium with one another at a particular temperature.
As the gas bubble forms within the liquid, the vapor pressure within the bubble must be equal to atmospheric pressure to escape the surface, at which point the liquid boils.
As the gas bubble forms within the liquid, the vapor pressure within the bubble must be equal to atmospheric pressure to escape the surface, at which point the liquid boils.

[SIZE=+1]The Macroscopic View[/SIZE]
The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); that is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample of the liquid (or solid) in a closed container.
Source: http://www.chem.purdue.edu/gchelp/liquids/vpress.html
Look pressure is being exerted source for this pic is http://www.chem.ufl.edu/~itl/2045/lectures/FG11_020.GIF
One more for good measure
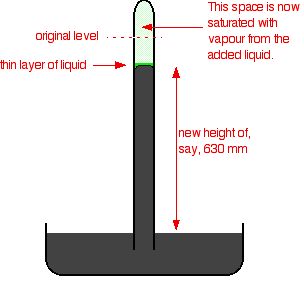
Words to go with it: [FONT=Helvetica, Arial]The saturated vapour pressure of the liquid will force the mercury level down a bit. You can measure the drop - and this gives a value for the saturated vapour pressure of the liquid at this temperature. In this case, the mercury has been forced down by a distance of 760 - 630 mm. The saturated vapour pressure of this liquid at the temperature of the experiment is 130 mmHg.
source: http://www.chemguide.co.uk/physical/phaseeqia/vapourpress.html
.
Last edited:
What always helped me conceptualize vapor pressure was thinking of it in terms of volatility. Something that is relatively volatile will exert a greater vapor pressure, and if an open system, will evaporate more quickly because the particles tend to escape the liquid and aerosolize. Vapor pressure is temperature dependent, where increasing temperature typically yields greater vapor pressure. Water always has a vapor pressure associated with it (unless it's at 0K, which is, so far, experimentally impossible to obtain), and this is why you lose water to evaporation if it is an open system after time. Boiling is a relative term, and it is technically the term that describes the event where the vapor pressure of a liquid is equal to atmospheric pressure, by definition. Boiling on Earth is not the same as boiling on the Moon because the atmospheric pressures are different. So, just know that boiling, by definition, is the event at which the vapor pressure of a liquid is equal to (or even greater if you have a large enough temperature spike) atmospheric pressure.
I am trying to understand why it is that vapor pressure MUST be equal to atmospheric pressure, like on a microscopic/macrosopic level what is going on? Vapor pressure is too "weak" to overcome the atmospheric pressure? I don't get it though because it's not vapor pressure till its in the vapor phase, and by that time it's not exactly competing against the "atmospheric pressure"
It might help you to draw it as a force diagram
|
|
|
V (downward force of atmosphere)
___________________________________________ (water/air interphase)
^ (upward force of liquid via vapor pressure)
|
|
|
|
Since pressure = F/A, and we'll just assume area is a constant, we can look at Force itself, alone. When atmospheric pressure > vapor pressure, there is a net force for the atmosphere to push down on the water, leading to relatively small liberation of water vapor. When vapor pressure >= atmospheric pressure, the water vapor can do as it pleases because there is no net force pushing down on it.
Vapor pressure in a CLOSED system should be thought of a little differently. In this case, you have a liquid at a certain temperature, no air on the top (vacuum off the air on the top), and you are measuring the pressure that is above the liquid produced by the molecules that have been liberated from the liquid (and this is one way in which you can experimentally determine the intrinsic vapor pressure of the liquid at a specific temperature). And, according to PV=nRT, you have a constant volume, constant temperature, so P is directly proportional to n, the number of moles in the gas phase. The more moles = the more pressure.
|
|
|
V (downward force of atmosphere)
___________________________________________ (water/air interphase)
^ (upward force of liquid via vapor pressure)
|
|
|
|
Since pressure = F/A, and we'll just assume area is a constant, we can look at Force itself, alone. When atmospheric pressure > vapor pressure, there is a net force for the atmosphere to push down on the water, leading to relatively small liberation of water vapor. When vapor pressure >= atmospheric pressure, the water vapor can do as it pleases because there is no net force pushing down on it.
Vapor pressure in a CLOSED system should be thought of a little differently. In this case, you have a liquid at a certain temperature, no air on the top (vacuum off the air on the top), and you are measuring the pressure that is above the liquid produced by the molecules that have been liberated from the liquid (and this is one way in which you can experimentally determine the intrinsic vapor pressure of the liquid at a specific temperature). And, according to PV=nRT, you have a constant volume, constant temperature, so P is directly proportional to n, the number of moles in the gas phase. The more moles = the more pressure.
Last edited:
That's how I was trying to think about it...but is it still fair to call it "Vapor Pressure" if it's still in the liquid phase? That's what I'm not getting I guess. Doesn't the vapor pressure of the gas exert itself onto the liquid phase?
Yes, a liquid will usually always have a vapor pressure on top of it in a closed system. And yes, once equilibrium has been reached between the two phases, the vapor pressure is exerted from the gas throughout the entire volume above the liquid. The vapor pressure exerts pressure onto the liquid and container it is contained in.
Last edited:
All the posters have made pretty good points, but I think I have the answer that you are looking for.
Vapor pressure is defined as the pressure exerted by the gas above a liquid in a closed container (where there was vacuum initially). The gas is, of course, the same substance as a liquid, and this is the only gas in this container. This means that (for sufficiently volatile liquids), some of the substance is in gas phase. This is because there is always an equilibrium between liquid and gas, written as
A(l) ->/<- A(g).
Where the position of the equilibrium lies varies with temperature, with higher temperature favoring gas. Remember that one way of measuring concentration of gas is pressure. If you think about the above as a reaction, you can use Kp = P(gas) to assign a value to the position of the equilibrium. But P(gas) is precisely the definition of vapor pressure, so vapor pressure is really an equilibrium constant for the phase change between the liquid and the gas form at a specific temperature.
If you increase temperature, the equilibrium constant, P(gas), and vapor pressure (all equivalent) increase. In a closed container, that simply means that more of the liquid will be converted to gas. In other words, a larger portion of the substance is in the gas phase as temperature is increased. At some point, as vapor pressure becomes too much, the container might break, or we might reach a supercritical fluid. Now imagine, though, that we are not talking about a closed container but an open container. All open containers are exposed to the atmospheric pressure. Atmospheric pressure is the pressure of all the gases above the liquid. The vapor pressure of the liquid (the pressure due to the gaseous form of the liquid) cannot be higher than atmospheric pressure, because now vapor is free to escape. At a temperature higher than the boiling point of a liquid, the vapor pressure is supposed to be higher than atmospheric pressure, which is not possible in an open system. Hence the liquid cannot go to a higher temperature than its boiling point, because that would imply that it is in equilibrium with its vapor, whose exerted pressure is greater than the atmospheric pressure. At this temperature, then, all of the liquid boils (bubbles form when this happens rapidly).
This also explains how evaporation can occur. If a cup of water is exposed to air, then it will eventually evaporate away, even at room temperature. The equilibrium between liquid water and water vapor favors liquid water, and the vapor pressure at room temperature is quite low. However, it's not 0, so there is some amount of vapor in equilibrium with the liquid. This vapor is free to escape, but if it does, then there is now less vapor in equilibrium with the liquid. Per Le Chatelier's principle, more liquid needs to evaporate to reach equilibrium, which results in more vapor escaping, and so on. Think of boiling and evaporation as an equilibrium reaction in which the product is continuously taken out of the system.
Vapor pressure is defined as the pressure exerted by the gas above a liquid in a closed container (where there was vacuum initially). The gas is, of course, the same substance as a liquid, and this is the only gas in this container. This means that (for sufficiently volatile liquids), some of the substance is in gas phase. This is because there is always an equilibrium between liquid and gas, written as
A(l) ->/<- A(g).
Where the position of the equilibrium lies varies with temperature, with higher temperature favoring gas. Remember that one way of measuring concentration of gas is pressure. If you think about the above as a reaction, you can use Kp = P(gas) to assign a value to the position of the equilibrium. But P(gas) is precisely the definition of vapor pressure, so vapor pressure is really an equilibrium constant for the phase change between the liquid and the gas form at a specific temperature.
If you increase temperature, the equilibrium constant, P(gas), and vapor pressure (all equivalent) increase. In a closed container, that simply means that more of the liquid will be converted to gas. In other words, a larger portion of the substance is in the gas phase as temperature is increased. At some point, as vapor pressure becomes too much, the container might break, or we might reach a supercritical fluid. Now imagine, though, that we are not talking about a closed container but an open container. All open containers are exposed to the atmospheric pressure. Atmospheric pressure is the pressure of all the gases above the liquid. The vapor pressure of the liquid (the pressure due to the gaseous form of the liquid) cannot be higher than atmospheric pressure, because now vapor is free to escape. At a temperature higher than the boiling point of a liquid, the vapor pressure is supposed to be higher than atmospheric pressure, which is not possible in an open system. Hence the liquid cannot go to a higher temperature than its boiling point, because that would imply that it is in equilibrium with its vapor, whose exerted pressure is greater than the atmospheric pressure. At this temperature, then, all of the liquid boils (bubbles form when this happens rapidly).
This also explains how evaporation can occur. If a cup of water is exposed to air, then it will eventually evaporate away, even at room temperature. The equilibrium between liquid water and water vapor favors liquid water, and the vapor pressure at room temperature is quite low. However, it's not 0, so there is some amount of vapor in equilibrium with the liquid. This vapor is free to escape, but if it does, then there is now less vapor in equilibrium with the liquid. Per Le Chatelier's principle, more liquid needs to evaporate to reach equilibrium, which results in more vapor escaping, and so on. Think of boiling and evaporation as an equilibrium reaction in which the product is continuously taken out of the system.
- Joined
- Jun 27, 2007
- Messages
- 7,545
- Reaction score
- 196
Thread moved. This type of thread belongs in the Study Q&A forum.
- Joined
- Aug 29, 2012
- Messages
- 513
- Reaction score
- 107
Sorry to bring this thread back but tbr says "the BP of a liquid is increased by increasing the atmospheric pressure above the liquid. This can be accomplished by vaporizing the liquid in a closed system."
( page 83 in chem chap 7)
How is this increasing the atmospheric pressure? Isn't this just increasing the vapor pressure? Is there even atmospheric pressure in a closed system?
( page 83 in chem chap 7)
How is this increasing the atmospheric pressure? Isn't this just increasing the vapor pressure? Is there even atmospheric pressure in a closed system?
Last edited:
- Joined
- Dec 26, 2008
- Messages
- 5,436
- Reaction score
- 4,042
Sorry to bring this thread back but tbr says "the BP of a liquid is increased by increasing the atmospheric pressure above the liquid. This can be accomplished by vaporizing the liquid in a closed system."
( page 83 in chem chap 7)
How is this increasing the atmospheric pressure? Isn't this just increasing the vapor pressure? Is there even atmospheric pressure in a closed system?
Perhaps, in closed systems, the vapor pressure acts in a similar fashion to the atmospheric pressure in that it exerts force of the liquids surface resisting further molecules from escaping the liquid phase into the gas phase. That's only a speculation and I could be wrong.
- Joined
- Aug 29, 2012
- Messages
- 513
- Reaction score
- 107
Ok that makes sense.. In general though vapor pressure sort of exerts its force away from the surface right? Not towards the surface?
- Joined
- Jul 9, 2012
- Messages
- 9,248
- Reaction score
- 8,719
I always think of it this way: evaporation is what happens when the individual liquid molecules have enough energy to 'escape' the liquid. In any given sample of liquid, some molecules will be moving faster than others, and a few of the faster ones will have enough energy to be vapor above the liquid. Thus, a sample of liquid exerts a vapor pressure on the space above it because some number of its atoms are bouncing around in that space in gas form.
The more heat in the liquid, the higher the average energy of the particles, and more of those particles will end up with enough energy to 'escape/evaporate' and so the vapor pressure will be higher.
A boiling point, by definition, is the point at which the average energy level of the atoms is high enough to escape into gas. If you are in a closed system, the overall pressure of the container is 'forcing' the atoms together, so they can't escape. If you are in open air, it is the pressure of the atmosphere which the atoms must overcome to escape liquid form. Therefore, the liquid will only be able to boil - have its atoms escape into the air - if they can push against this pressure. The pushing that they do is the vapor pressure, and so liquid can only boil when its vapor pressure is at least as high as the atmospheric pressure.
The more heat in the liquid, the higher the average energy of the particles, and more of those particles will end up with enough energy to 'escape/evaporate' and so the vapor pressure will be higher.
A boiling point, by definition, is the point at which the average energy level of the atoms is high enough to escape into gas. If you are in a closed system, the overall pressure of the container is 'forcing' the atoms together, so they can't escape. If you are in open air, it is the pressure of the atmosphere which the atoms must overcome to escape liquid form. Therefore, the liquid will only be able to boil - have its atoms escape into the air - if they can push against this pressure. The pushing that they do is the vapor pressure, and so liquid can only boil when its vapor pressure is at least as high as the atmospheric pressure.
- Joined
- Jul 9, 2012
- Messages
- 9,248
- Reaction score
- 8,719
Ok that makes sense.. In general though vapor pressure sort of exerts its force away from the surface right? Not towards the surface?
Pressure, with gases, is an effect of atoms bouncing off of surfaces. The more atoms you have (the more mols of gas) and the more energy those atoms have, the higher the pressure. The pressure is equal throughout any given system; if you blow up a balloon, you are increasing the pressure inside the balloon and the air pushes on all surfaces of the balloon from the inside.
So when some of the atoms of a liquid have enough energy to escape as a gas, they join the atoms of air above. In a closed system, say a flask with the lid on it, those escaped atoms will cause the pressure inside the flask. If you think of it as the push of all the bouncing gas atoms, you'll see that it's not a unidirectional force.
In an open air situation, you aren't increasing the overall atmospheric pressure, because your sample is just too small compared to the entire atmosphere.
- Joined
- Aug 29, 2012
- Messages
- 513
- Reaction score
- 107
Okay thanks so much!! Great answer.
Similar threads
- Replies
- 2
- Views
- 2K
- Replies
- 2
- Views
- 2K
D
- Replies
- 6
- Views
- 1K
