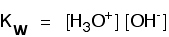- Joined
- May 22, 2004
- Messages
- 1,285
- Reaction score
- 1
what is the acid dissociation constant ka of water?
the answer is 1.8e-16, but how would you get it?
I first thought it is 1.0e-7, but apparently I am wrong.
the answer is 1.8e-16, but how would you get it?
I first thought it is 1.0e-7, but apparently I am wrong.