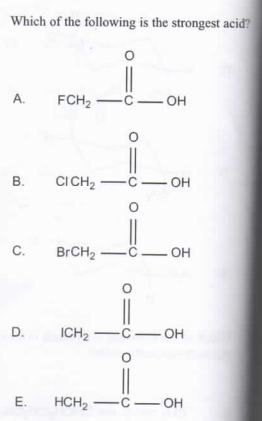
F is a strong withdrawing group, it pulls all the electrons towards itself. This means that the H is not strongly pulled, and can easily be taken away by a base. A strong acid is one that readily/easily gives up its H, and since F doesn't care very much about the H (it's the most electronegative, it loooooves electrons, not protons), (A) is the most acidic. Hope this helps 🙂