- Joined
- Jun 14, 2014
- Messages
- 62
- Reaction score
- 37
Hey guys. Hope your MCAT studying is going well! I was hoping one of you might know the reason behind the following phenomenon:
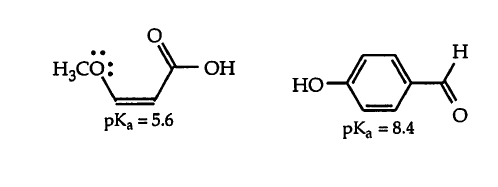
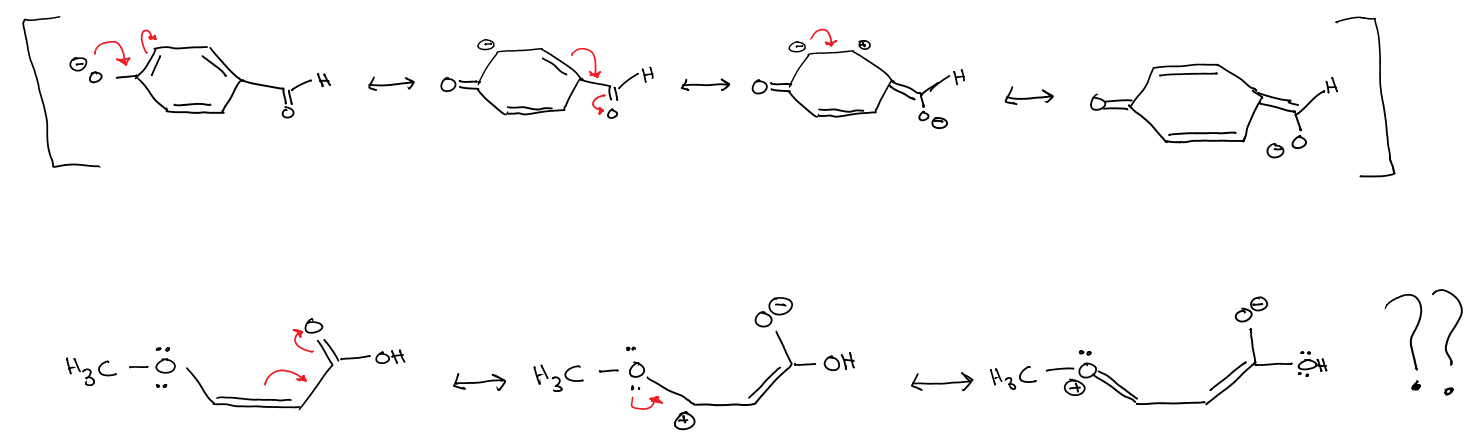
One of these oxygens acts as an electron-withdrawing group whereas the other acts as an electron-donating group. But both have lone pairs of electrons to donate to the resonance structures. But they still have idiosyncratic effects?
The only reason I could think of, and it doesn't seem very convincing, is that the lone pairs of electrons in the ether are right beside the pi-bond. So they can directly donate electron density into the resonance delocalization. In the p-hydroxybenzaldehyde however, there is no adjacent pi-bond to donate the lone pair to? And oxygen being electronegative, it causes the alpha carbon to attain a partial positive charge, which acts as an electron-withdrawing group from the resonance structure. Any input would be greatly appreciated! Thank you

One of these oxygens acts as an electron-withdrawing group whereas the other acts as an electron-donating group. But both have lone pairs of electrons to donate to the resonance structures. But they still have idiosyncratic effects?
The only reason I could think of, and it doesn't seem very convincing, is that the lone pairs of electrons in the ether are right beside the pi-bond. So they can directly donate electron density into the resonance delocalization. In the p-hydroxybenzaldehyde however, there is no adjacent pi-bond to donate the lone pair to? And oxygen being electronegative, it causes the alpha carbon to attain a partial positive charge, which acts as an electron-withdrawing group from the resonance structure. Any input would be greatly appreciated! Thank you