- Joined
- Dec 18, 2015
- Messages
- 3,216
- Reaction score
- 4,930
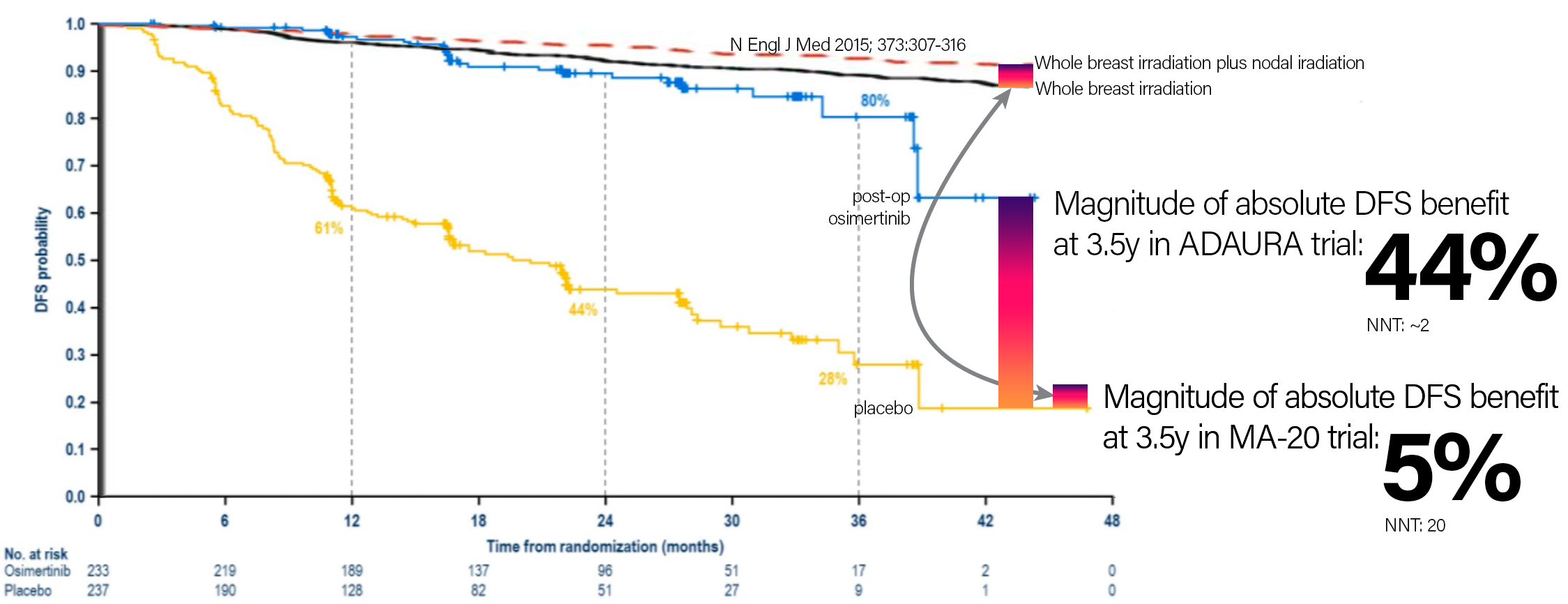
Off-top-of-head impressions 'bout ADAURA trial. From one rad onc's perspective.
1) This makes surgery more likely, radiation less likely, in the minds of many treating physicians for lung cancer (EGFR mutated lung ca). Won't be huge shifts, but anyone with an imagination can see all the trend lines.
2) Osi costs about $200K/year from what I've heard. This is 3 years of therapy after surgery. So at least $600K per patient. Holy moly.
3) People harping on the "oligometastatic paradigm" (saving rad onc) need be mindful that this paradigm gets robbed by successes with drugs like osi.
4) The real question: would anyone think about or push for osi after lung SBRT for the right patient?
But for real: the oncological success some of these drugs are having... I'm very jealouse.
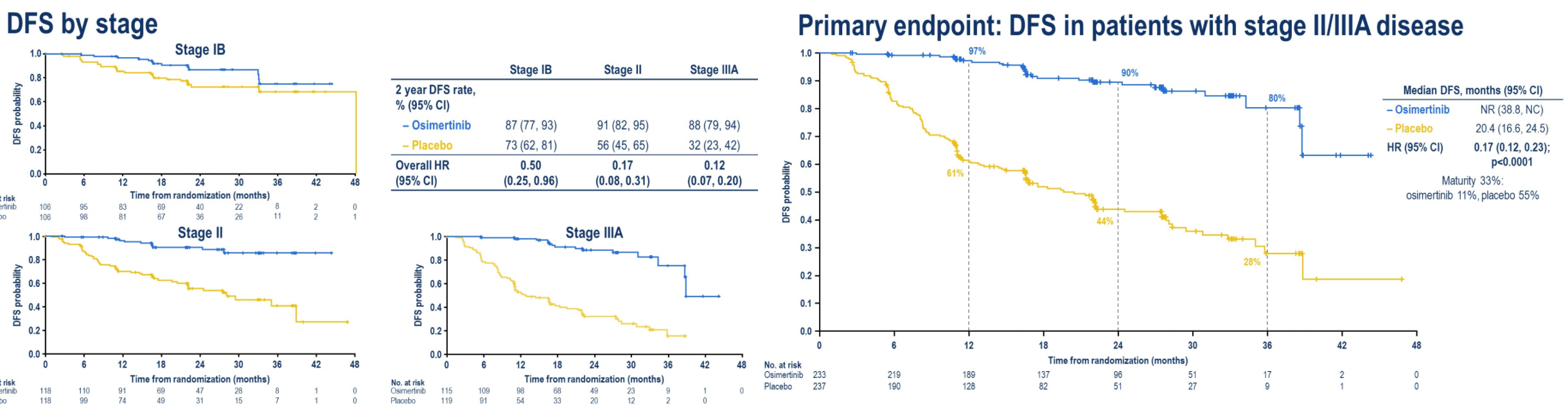
1) This makes surgery more likely, radiation less likely, in the minds of many treating physicians for lung cancer (EGFR mutated lung ca). Won't be huge shifts, but anyone with an imagination can see all the trend lines.
2) Osi costs about $200K/year from what I've heard. This is 3 years of therapy after surgery. So at least $600K per patient. Holy moly.
3) People harping on the "oligometastatic paradigm" (saving rad onc) need be mindful that this paradigm gets robbed by successes with drugs like osi.
4) The real question: would anyone think about or push for osi after lung SBRT for the right patient?
But for real: the oncological success some of these drugs are having... I'm very jealouse.