- Joined
- Oct 19, 2015
- Messages
- 667
- Reaction score
- 389
- Points
- 5,306
- Dental Student
Which is more stable, a carbocation bonded to two methyl groups, or bonded to one methyl group and one phenyl group?
Put in a different way, in the image I've attached, will there be a hydride shift to produce a more stable carbocation or no?
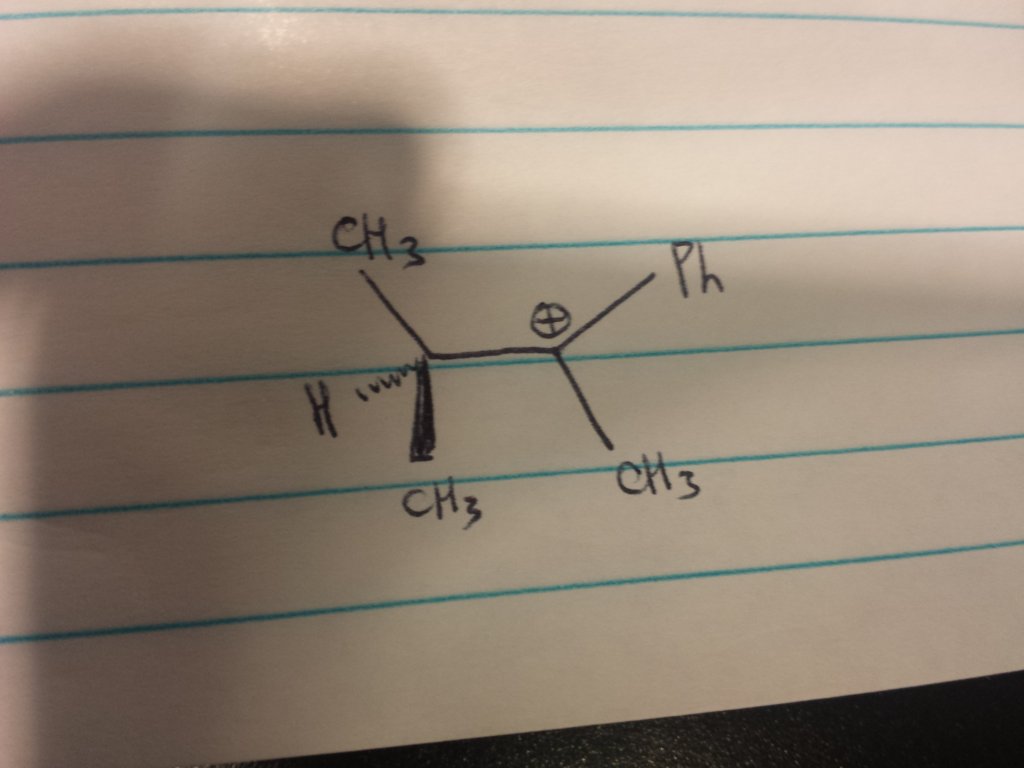
Put in a different way, in the image I've attached, will there be a hydride shift to produce a more stable carbocation or no?
