- Joined
- Aug 25, 2013
- Messages
- 24
- Reaction score
- 0
- Points
- 51
- Pre-Medical
Advertisement - Members don't see this ad
From Mprep question of the day
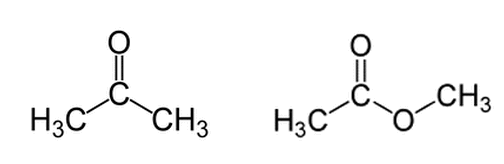
Acetone (on the left) and methyl acetate (on the right) are shown below. Regarding deprotonation of a hydrogen from the left methyl group (for both molecules), which molecule will have the higher pKa and why?
A
The molecule on the left will have the higher pKa and destabilize the conjugate base
B
The molecule on the left will have the higher pKa and stabilize the conjugate base
C
The molecule on the right will have the higher pKa and destabilize the conjugate base
D
The molecule on the right will have the higher pKa and stabilize the conjugate base
Answer is C. I thought the answer would be D. I don't understand how methyl acetate is destabilized upon loss of a proton. Isn't there more resonance once it is deprotonated so more stabilized?
Acetone (on the left) and methyl acetate (on the right) are shown below. Regarding deprotonation of a hydrogen from the left methyl group (for both molecules), which molecule will have the higher pKa and why?

A
The molecule on the left will have the higher pKa and destabilize the conjugate base
B
The molecule on the left will have the higher pKa and stabilize the conjugate base
C
The molecule on the right will have the higher pKa and destabilize the conjugate base
D
The molecule on the right will have the higher pKa and stabilize the conjugate base
Answer is C. I thought the answer would be D. I don't understand how methyl acetate is destabilized upon loss of a proton. Isn't there more resonance once it is deprotonated so more stabilized?

