- Joined
- Feb 26, 2014
- Messages
- 201
- Reaction score
- 57
- Points
- 4,671
- Location
- Columbus, OH
- Dental Student
I understand that polar bonds can be included in an overall nonpolar molecule.
CF4 is tetrahedral and a nonpolar molecule with polar bonds.
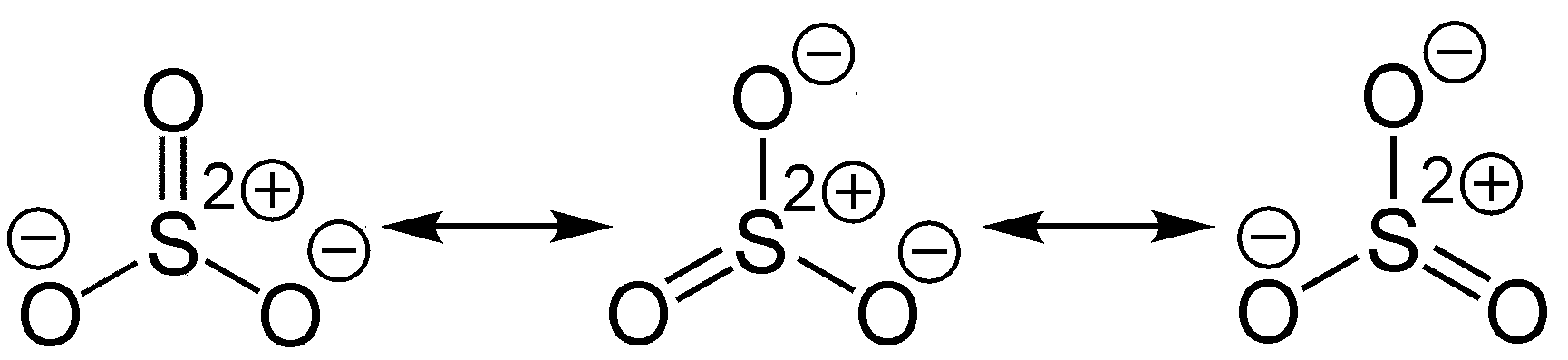
What about SO3? It should be Sulfer double bonded to Oxygen in a trigonal planar arrangement.
Am I missing something? To me both A/D are correct.
13. Which of the following is an example of a compound that has polar bonds but a non-polar shape?
There are two different types of polarity that have to be understood to answer this question. They are bond polarity and molecular polarity. For bond polarity, if there is a large difference in the electronegativities of the elements that make up a bond, then the elements are most likely sharing the electrons in the bond unequally, resulting in a polar bond. However, molecular polarity deals with the shape of the molecule, looking for symmetry. Symmetry leads to a non-polar molecule. The only molecules in the answer options that have a symmetrical shape are CF4 and N2. However, N2 would have non-polar bonds as the N-N bond would have the same electronegativity and would be sharing the electrons equally. Therefore, CF4 must be the answer as the electronegativities vary with the C and the F making the C-F bonds polar.
Topic: Liquids and Solids
CF4 is tetrahedral and a nonpolar molecule with polar bonds.
What about SO3? It should be Sulfer double bonded to Oxygen in a trigonal planar arrangement.
Am I missing something? To me both A/D are correct.
13. Which of the following is an example of a compound that has polar bonds but a non-polar shape?
- 1. A. SO3
- 2. B. H2O
- 3. C. N2
- 4. D. CF4
- 5. E. NH3
There are two different types of polarity that have to be understood to answer this question. They are bond polarity and molecular polarity. For bond polarity, if there is a large difference in the electronegativities of the elements that make up a bond, then the elements are most likely sharing the electrons in the bond unequally, resulting in a polar bond. However, molecular polarity deals with the shape of the molecule, looking for symmetry. Symmetry leads to a non-polar molecule. The only molecules in the answer options that have a symmetrical shape are CF4 and N2. However, N2 would have non-polar bonds as the N-N bond would have the same electronegativity and would be sharing the electrons equally. Therefore, CF4 must be the answer as the electronegativities vary with the C and the F making the C-F bonds polar.
Topic: Liquids and Solids