Hey guys, I was wondering if any of you noticed that there's a bootcamp error on the Orgo exam # 4 (Question 5)
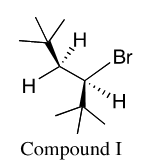
It says that this compound undergoes E2 reaction and that it is best described by "The lease stable antiperiplanar conformation that leads to the formation of the minor product"
While this is the least STABLE conformation due to the tertbutyl groups both on the wedge, there is only 1 Beta Hydrogen, leading this "least stable" conformation to the MAJOR product.
If I am wrong, someone please tell me. haha
It says that this compound undergoes E2 reaction and that it is best described by "The lease stable antiperiplanar conformation that leads to the formation of the minor product"
While this is the least STABLE conformation due to the tertbutyl groups both on the wedge, there is only 1 Beta Hydrogen, leading this "least stable" conformation to the MAJOR product.
If I am wrong, someone please tell me. haha
