- Joined
- Oct 8, 2014
- Messages
- 13
- Reaction score
- 6
I was working on the Bootcamp section specific tests and I ran into this:
3. Calcium has a larger atomic radius than magnesium because of the:
E. Increase in electron shielding
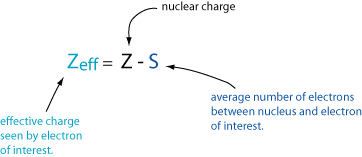
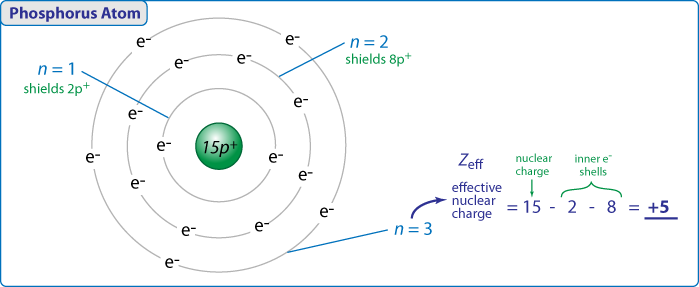
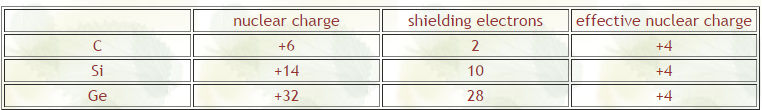
First, almost all of the periodic trends can be derived from the two concepts of effective nuclear charge and electron shielding. In a given group in the periodic table, the effective nuclear charge stays relatively constant due to the constant number of valence electrons; the increasing number of protons is balanced with an increasing number of core electrons. The effective nuclear charge is responsible for the decrease in atomic size across periods.
The electron shielding effect reduces the effect of a full nuclear charge. As more core electrons are added, they begin to “shield” the valence electrons from the increasingly positive nucleus. This allows the outer electrons to move farther away from the nucleus, hence increasing atomic size. The electron shielding effect is responsible for the increase in atomic size moving down groups.
Topic: Periodic Properties and Trends
So they said that going down a column, the electron shielding effect is responsible for the increased size and that going right in a row, the effective nuclear charge is responsible for the decrease in size. I'm confused about this because I always thought that even though when you go down a column, it is true that you will have more electron shielding (more orbitals are filled) but don't you also have more protons in the nucleus? So with the same number of valence electrons, you would be further out but there also will be a stronger charge? Thanks for any input!
3. Calcium has a larger atomic radius than magnesium because of the:
E. Increase in electron shielding
First, almost all of the periodic trends can be derived from the two concepts of effective nuclear charge and electron shielding. In a given group in the periodic table, the effective nuclear charge stays relatively constant due to the constant number of valence electrons; the increasing number of protons is balanced with an increasing number of core electrons. The effective nuclear charge is responsible for the decrease in atomic size across periods.
The electron shielding effect reduces the effect of a full nuclear charge. As more core electrons are added, they begin to “shield” the valence electrons from the increasingly positive nucleus. This allows the outer electrons to move farther away from the nucleus, hence increasing atomic size. The electron shielding effect is responsible for the increase in atomic size moving down groups.
Topic: Periodic Properties and Trends
So they said that going down a column, the electron shielding effect is responsible for the increased size and that going right in a row, the effective nuclear charge is responsible for the decrease in size. I'm confused about this because I always thought that even though when you go down a column, it is true that you will have more electron shielding (more orbitals are filled) but don't you also have more protons in the nucleus? So with the same number of valence electrons, you would be further out but there also will be a stronger charge? Thanks for any input!