Buffers got me sooooo f'in bad, but then all of a sudden it just clicked for me this semester.
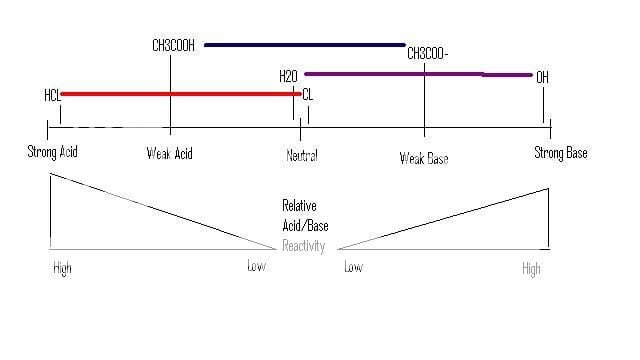
So, HCl is a super strong acid, meaning, in water with a normal pH, Cl is going to ditch the H at whatever chance it gets to make H3O(Hydronium). HCl is a strong acid because Cl is so STABLE, HAPPY, FINE, etc, by it's lonesome. Therefore, something that is so STABLE, HAPPY, FINE, etc by itself, will make a ****ty base even in its negative form, cause it doesnt want it's H back.
NaOH is a super strong base, meaning, in water, that OH is going to suck up every H it can get it's hands one. OH negative is extremely UNSTABLE, UNHAPPY,NOT FINE, etc by it's self. Thats what makes it a strong base, so when its made happy by taking up and H and effectively making H2O, its so god damn happy to be with that H it's not going to let it go, effectively making a terrible acid.
Now, to the weak acids...Depending on the conditions they are are...they are kinda just "meh." For the sake of simplicity, we are going to assume this compound I am going to use (Acetic Acid [CH3COOH]) is in the dead center on the Acidic and the neutral range. The conjugate base of Acetic Acid (Acetate [CH3COO-]) is the complete opposite, meaning its in the dead center of the neutral and BASIC range.
So, we throw Acetic Acid in regular water, what happens? On a large scale...nothing (a VERY VERY small amount of CH3COOH will ditch it's H). This is chalked up due to the fact that Acetic Acid is kinda like "...Ya, im pretty happy with my H, ill keep it." And water is like, "Ya dude, you are not strong enough to force your H on me, and also cause im a pretty ****ty base.(H2O)"
Then, we drop in some OH- in the form of NaOH (it dissociates and we dont care about Na+). OH- is running around screaming "GIVE ME ALL YOUR MOTHER #$@%in H's!" Now it has a choice, Acetic Acid, or H2O. Acetic acid is a weak acid, but its a stronger acid than H2O, so Acetic Acid is going to Let loose all of the H's it has in the amount equal to the OH- in the solution.
Assuming the amount of OH- is lesser than the amount of Acetic Acid we will now have Acetic acid and Acetate in solution.
You may be wondering, why doesnt Acetate take up an H and go back to Acetic Acid...Well go back to my statement where I said, "(Acetic Acid [CH3COOH]) is in the dead center on the Acidic and the neutral range. The conjugate base of Acetic Acid (Acetate [CH3COO-]) is the complete opposite, meaning its in the dead center of the neutral and BASIC range." So when Acetic Acid was saying, "Ya water, Im pretty happy with H, ill stick with it." The new Acetate is now saying, "ya guys, i was pretty happy with that H, but ya know, im just as fine without it, so ill chill."
Im really sorry for the corney dialoge, but thats how I teach me study group. He's an image that I hope will send all this home for ya.
For a weak base, its completely the reverse of everything I just said.