So I saw a question about the C5 position on a cytosine molecule, and I know how to find that now, but I'm blanking on how to determine the position of each atom in a molecule in general. How to do you know which atom to start counting from and which direction to count in?
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
C5 Positon on a Cytosine Molecule
- Thread starter gea113
- Start date
- Joined
- Jun 17, 2014
- Messages
- 63,099
- Reaction score
- 154,727
So I saw a question about the C5 position on a cytosine molecule, and I know how to find that now, but I'm blanking on how to determine the position of each atom in a molecule in general. How to do you know which atom to start counting from and which direction to count in?
Here is the structure of cytosine:
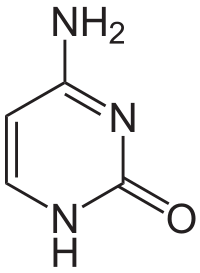
Cytosine itself consists of 4 carbon atoms, so I don't know where you got C5 position. Are you confusing cytosine for cytidine?
Here is the structure of cytosine:

Cytosine itself consists of 4 carbon atoms, so I don't know where you got C5 position. Are you confusing cytosine for cytidine?

OP is following the rules for numbering atoms in a ring. The first letter gives the nature of the atom (carbon) and the second letter gives its position (5). So the question is actually: what is the 5-position on this ring? As in 5-methylcytosine.
The rules are often long and convoluted so I'll direct OP to the following link: What is the reason behind the numbering of nitrogen bases?
- Joined
- Jun 17, 2014
- Messages
- 63,099
- Reaction score
- 154,727
OP is following the rules for numbering atoms in a ring. The first letter gives the nature of the atom (carbon) and the second letter gives its position (5). So the question is actually: what is the 5-position on this ring? As in 5-methylcytosine.
The rules are often long and convoluted so I'll direct OP to the following link: What is the reason behind the numbering of nitrogen bases?
Yeah i'm familiar with the heterocycle naming conventions but for the sake of consistency, C5 is misleading because it looks as if cytosine had 5 carbons when it only has 4. Framing the question in terms of what's the 5th position? or how do I number the atoms in the heterocycle? is much clearer.
Yeah i'm familiar with the heterocycle naming conventions but for the sake of consistency, C5 is misleading because it looks as if cytosine had 5 carbons when it only has 4. Framing the question in terms of what's the 5th position? or how do I number the atoms in the heterocycle? is much clearer.
OP is using standard chemical conventions when it comes to naming and while this may be unclear to you, it is the widely accepted use in the field. Understanding the language of chemistry is just as important as understanding its tenets. For an example of an actual use of this term in context, see this article from J Biol Chem that talks about an enzyme's C5 methyltransferase activity: https://www.researchgate.net/profil...NA-Cytosine-C5-Methyltransferase-Activity.pdf
- Joined
- Jun 17, 2014
- Messages
- 63,099
- Reaction score
- 154,727
OP is using standard chemical conventions when it comes to naming and while this may be unclear to you, it is the widely accepted use in the field. Understanding the language of chemistry is just as important as understanding its tenets. For an example of an actual use of this term in context, see this article from J Biol Chem that talks about an enzyme's C5 methyltransferase activity: https://www.researchgate.net/profil...NA-Cytosine-C5-Methyltransferase-Activity.pdf
Just out of curiosity, is heterocycle naming convention even tested on the MCAT? I didn't find it in the content guidelines and I don't recall its appearance anywhere. The best I can think of is being discussed in a passage, where details would provide clarity regarding how to name it.
Just out of curiosity, is heterocycle naming convention even tested on the MCAT? I didn't find it in the content guidelines and I don't recall its appearance anywhere. The best I can think of is being discussed in a passage, where details would provide clarity regarding how to name it.
It is not tested in the sense that you're not going to be asked "Number the carbon para to the ketone in cytosine." But it can appear in the context of a passage where, say, a paper like the one I linked is discussed and they talk about C5 methyltransferases. In that case, you better be able to deduce what a C5 methyltransferase is and not get confused about the carbon numbering since there are only four carbon atoms in the nucleobase.
Here is the structure of cytosine:

Cytosine itself consists of 4 carbon atoms, so I don't know where you got C5 position. Are you confusing cytosine for cytidine?

The image below shows the methylation of cytosine, where a methyl group is placed at C5 or position 5, if you count starting from the NH. So, the count goes NH => position 1, O=> position 2, N=> position 3, NH2=> position 4, and the methyl group (H3C) => position 5. Cytosine has four carbons with a chemical formula of C4H5N30, while methylcytosine has five carbons and a chemical formula of C5H7N30. Finally, when counting positions in nitrogenous bases, the nitrogens are always given the lowest possible number.
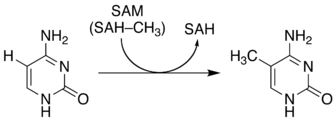
Image and information procured at the Wikepedia site using "5-Methylcytosine" as the search key.
Last edited:
Similar threads
- Replies
- 4
- Views
- 5K
- Replies
- 1
- Views
- 766
- Replies
- 1
- Views
- 2K
