How are heats of combustion used to compare relative stabilities of isomers?
If you have EK, its on page 30 of the organic book. Thanks!
If you have EK, its on page 30 of the organic book. Thanks!
How are heats of combustion used to compare relative stabilities of isomers?
If you have EK, its on page 30 of the organic book. Thanks!
Could you explain this more? "We can see that the enthalpy of the products will be greater for cyclohexane because we will generate more moles of them." How does cyclohexane generate more moles of them? Sorry it's been awhile since I've taken organic.
Thanks in advance!
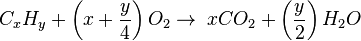
Hey,How are heats of combustion used to compare relative stabilities of isomers?
Hey ,
I seee #916 in random places, what does this mean? What are we dividing in this case to figure our the per CH2 energy released?
Thanks!
If you have EK, its on page 30 of the organic book. Thanks!
The heat of combustion (ΔHc) is the change in enthalpy of a combustion reaction when one mole of a compound undergoes complete combustion with oxygen.
Now since isomers have the exact same formula, but different bond to bond connectivity, we dont have to worry about different amounts of CO2 and H2O affecting the change in enthalpy. Therefore:
The larger the heat of combustion, the higher the energy level of the molecule and therefore the less stable the molecule!!!
This can be see if you look at the graph for an exothermic reaction!!
However, if we are comparing two different molecules of different sizes, for example cyclohexane vs cyclopropane. We can see that the enthalpy of the products will be greater for cyclohexane because we will generate more moles of them, and enthalpy is an extensive property. This still applies even though the sum of H for the reactants will be larger as well (the major contributor to the difference is the H for the products). From the formula:
ΔH = sum of H for products - sum of H for reactants
We see that we will have an overall greater ΔHc for cyclohexane. Does this mean that we cyclohexane is less stable than cyclopentane? No it doesn't!! Why is this the case? Well, first we know that cyclohexane has the lowest ring strain out of all the cycloalkanes. So how do we account for this difference? We account for this difference by having to take the ΔH in terms of "per CH2". Why do we do this you might ask? It's because we want to see the actual energy drop for each bond aka bond stabilitys on each CH2. We would divide our ΔH by the number of CH2 present in each ring. After doing this we can see that the ΔH per CH2 is actually less for cyclohexane than cyclopropane and and therefore the entire molecule has a greater stability than cyclopropane
