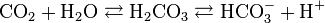- Joined
- Apr 14, 2014
- Messages
- 13
- Reaction score
- 0
Hello friends...I wanted to ask that in in hyperventilation when paco2 decreases and this equation shifts to left.
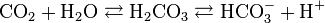 i read in literature that H+ and HCO3 combine to form carbonic acid and carbonic acid dissociates into co2 nad h2o as compensation.. due to hyperventilation and fall in paco2 ph level rises leading to alkalosis. My question is when H+ and Hco3 both combine to form carbonic acid and further then why they write just protons are lost and ph rises and causes alkalosis....why dosent loss of hco3 affect ph ..in my mind it was both are lost so ph shoudnt changee..please help me I can elaborate more if my question is not clear.....
i read in literature that H+ and HCO3 combine to form carbonic acid and carbonic acid dissociates into co2 nad h2o as compensation.. due to hyperventilation and fall in paco2 ph level rises leading to alkalosis. My question is when H+ and Hco3 both combine to form carbonic acid and further then why they write just protons are lost and ph rises and causes alkalosis....why dosent loss of hco3 affect ph ..in my mind it was both are lost so ph shoudnt changee..please help me I can elaborate more if my question is not clear.....