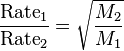ok, it says that Ne and He have the same kinetic energy (according to the kinetic molecular theory) but they do NOT have the same speed since Ne is heavier and therefore denser than He
but isn't kinetic energy directly proportional to speed?? forexample: catalysts act on the kinetics of the reaction by "speeding" up the rate, so an increase in kinetic energy causes increase in speed or motion of a molecule,
I don't understand why in that situation with the Ne and He they make the distinction between "kinetic energy" and "speed", if they have the same kinetic energy then they should have the same speed.
you might say they don't have the same speed because one is heavier than the other, well then if that's the case then they shouldn't have the same kinetic energy either yet they do,
so i guess iam asking...fill in the blank: if speed is weight dependent, then kinetic energy is ______dependent?
please help clarify this, thanks
but isn't kinetic energy directly proportional to speed?? forexample: catalysts act on the kinetics of the reaction by "speeding" up the rate, so an increase in kinetic energy causes increase in speed or motion of a molecule,
I don't understand why in that situation with the Ne and He they make the distinction between "kinetic energy" and "speed", if they have the same kinetic energy then they should have the same speed.
you might say they don't have the same speed because one is heavier than the other, well then if that's the case then they shouldn't have the same kinetic energy either yet they do,
so i guess iam asking...fill in the blank: if speed is weight dependent, then kinetic energy is ______dependent?
please help clarify this, thanks