- Joined
- Dec 24, 2014
- Messages
- 1,056
- Reaction score
- 290
- Points
- 5,276
- Dental Student
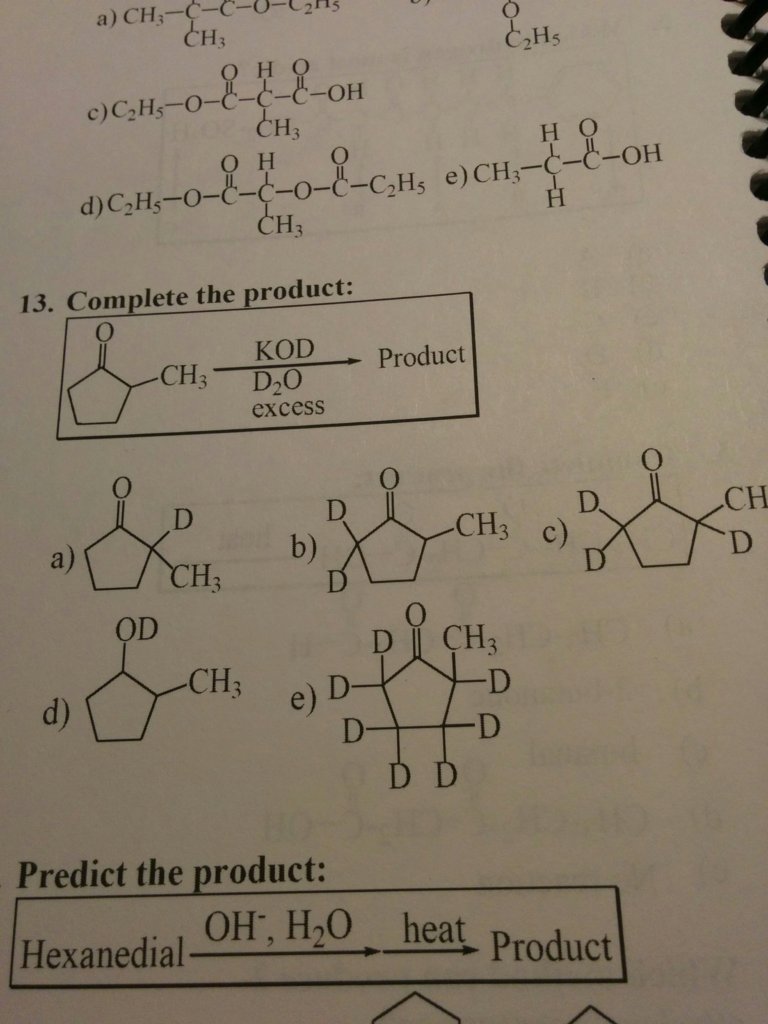
Do we always treat the D2O as a D-D or do we ever treat it like a D-OD (alcohol/water)? In the reaction pictured below, we treat it as a D2 and add to every alpha hydrogen. However, if we treated it as a D-OD like ch3oh wouldn't it only add to the most acidic hydrogen? Sorry I always get confused on these deuterion problems