- Joined
- Aug 16, 2006
- Messages
- 112
- Reaction score
- 0
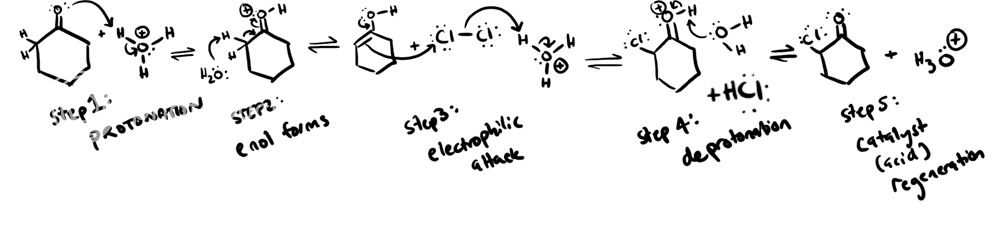
i think c=o is an activator and will o/p direct.
it does that because the hydrogen bonded to carbon next to carbony carbon is the most acidic and its easier to remove that. so Cl substitude that carbon. if it was a benzene ring I believe it would be meta directing
correct me if im wrong
yea this makes sense...hmm i'm confused now too so why does Cl add to that position? since it is acid catalyzed it shoudn't be pylling off an acidic H.What are you saying is pulling off the H? It is an acidic solution. this is what I don't understand. If it was Cl2 in OH-, then that would make sense. Since it's an acidic solution, it seems as though something would be protonated.