Move this to the MCAT discussion forum, please. Other people on there will find the answers useful.
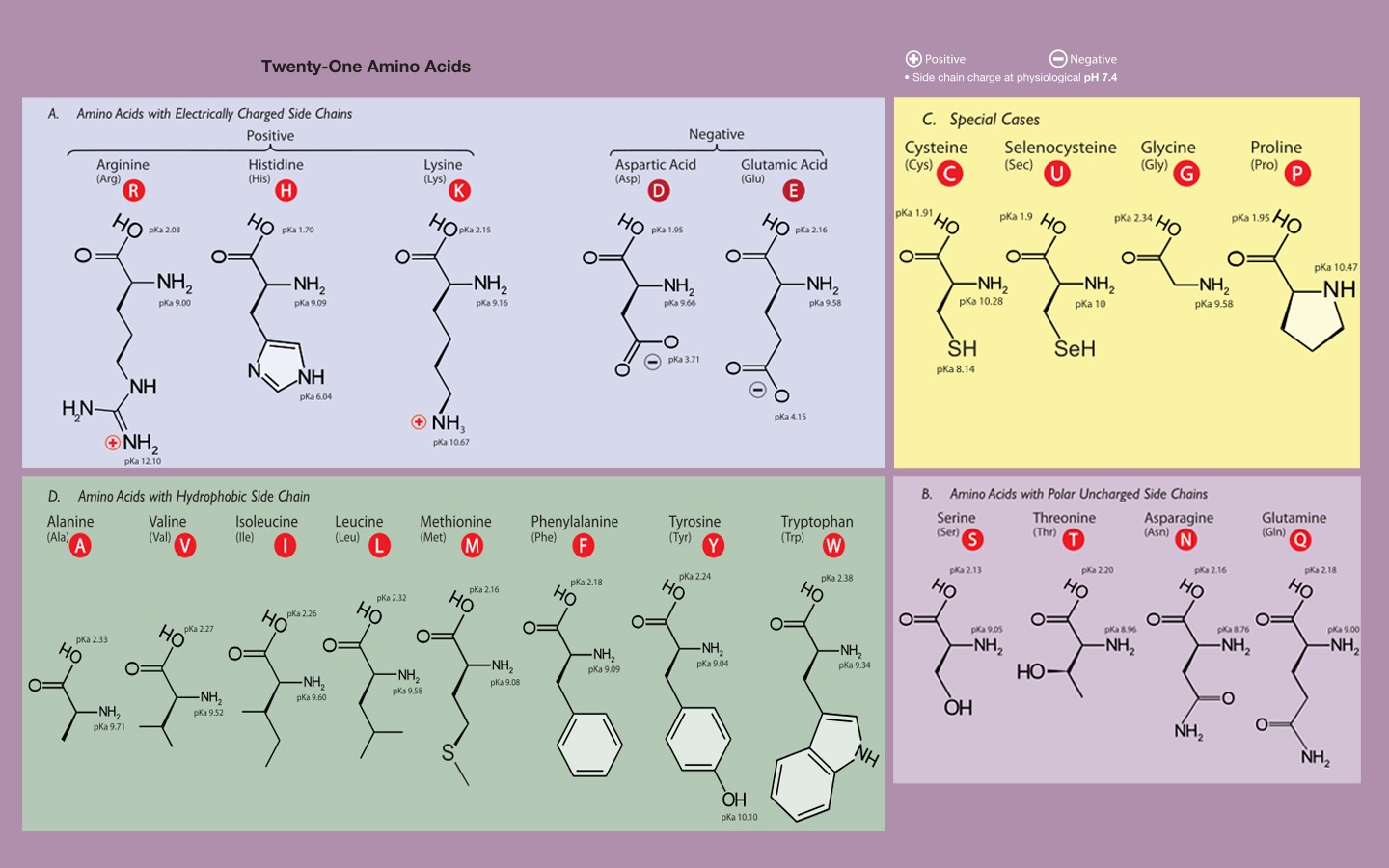
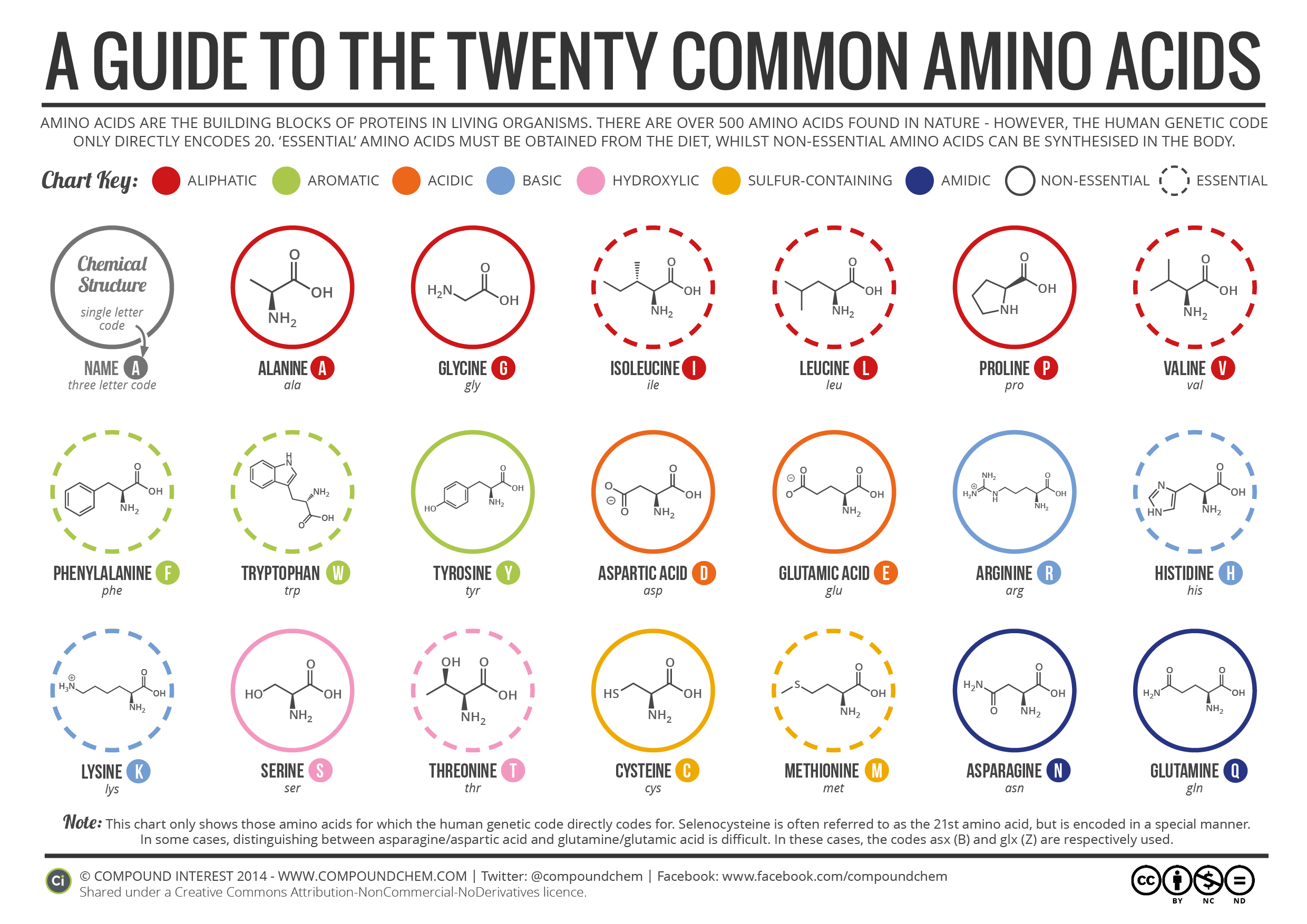
Here's what I think you should know. You should know the side chain structures well enough that you can answer a reasoning question from that knowledge. For example, replacement of valine with which of the following would least affect protein folding? A) P B) E C) W D) I. You should know that the pKa of the carboxy terminus is around 2 and the pKa of the amino terminus is around 9. The side chain pKas you should also be familiar with for the ones that have pKas that are relevant under normal conditions. So definitely D, E, H, K, and R since these are most often used in biology as ion-pairs and/or catalytic residues. Y and C are useful to know as well.
So if I asked you what the net charge of the following peptide is at pH 7, you should be able to calculate it: DEPGVILWYCMKRIGAH.
You don't have to memorize pIs but you should be able to calculate it if you're given the relevant equilibria and pKa values.