- Joined
- Jul 19, 2014
- Messages
- 47
- Reaction score
- 20
Hey SDN,
Today for the DAT quesiton of the day the following question was presented:
"Which reaction mechanism predominates in the reaction below?"
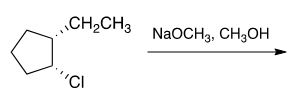
A. E1
B. E2
C. SN1
D. SN2
E. SN1 with rearrangement.
The answer has not been revealed yet but I am pretty confident that it is B: E2.
Strong, small base and and antiperiplanar position on the Cl and H make this possible.
Suppose the ethyl group was wedged (coming out of the page) rather than dashed as it is now. Would E2 still be the predominant reaction (not antiperiplanar anymore)? What about SN2, the Cl is on a secondary carbon which makes both E2 and SN2 favorable.
Any advice on how to differentiate these reactions quicker?
Thanks in advance!
Today for the DAT quesiton of the day the following question was presented:
"Which reaction mechanism predominates in the reaction below?"
A. E1
B. E2
C. SN1
D. SN2
E. SN1 with rearrangement.
The answer has not been revealed yet but I am pretty confident that it is B: E2.
Strong, small base and and antiperiplanar position on the Cl and H make this possible.
Suppose the ethyl group was wedged (coming out of the page) rather than dashed as it is now. Would E2 still be the predominant reaction (not antiperiplanar anymore)? What about SN2, the Cl is on a secondary carbon which makes both E2 and SN2 favorable.
Any advice on how to differentiate these reactions quicker?
Thanks in advance!
