Metals are extracted from their ores by a wide variety of techniques. An example of an extraction is of iron from its oxide, described by the following equation:
2Fe2O3 (s) + 3C (s) -----> 4Fe (s) + 3CO2 (g)
The relative ease of extraction of a metal from its oxide can be estimated using the Ellingham diagram, which is shown below. This diagram plots the the free energies of formation of various oxides per mole of consumed oxygen as a function of absolute temperature.
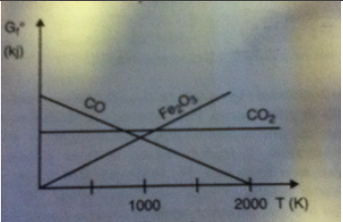
1.) Elemental carbon is most likely to reduce Fe2O3 in which of the following temperature ranges?
A. 500-1000 K
B. 500-1500K
C. Below 1000K
D. Above 1000K
2. When ferric oxide is produce to obtain iron metal, CO2 or CO can be produced. The production of carbon dioxide
A.) is directly proportional to the temp
B. is inversely proportional to the temp
C. is independent of the temp
D.) requires cooling of the CO.
Answer for both: D
2Fe2O3 (s) + 3C (s) -----> 4Fe (s) + 3CO2 (g)
The relative ease of extraction of a metal from its oxide can be estimated using the Ellingham diagram, which is shown below. This diagram plots the the free energies of formation of various oxides per mole of consumed oxygen as a function of absolute temperature.

1.) Elemental carbon is most likely to reduce Fe2O3 in which of the following temperature ranges?
A. 500-1000 K
B. 500-1500K
C. Below 1000K
D. Above 1000K
2. When ferric oxide is produce to obtain iron metal, CO2 or CO can be produced. The production of carbon dioxide
A.) is directly proportional to the temp
B. is inversely proportional to the temp
C. is independent of the temp
D.) requires cooling of the CO.
Answer for both: D
