- Joined
- May 23, 2014
- Messages
- 265
- Reaction score
- 37
For this question, I thought that dG = dH - TdS.
So for endothermic reactions, the dH has to be positive, and if it is spontaneous, the dG has to be negative.
Then dS has to be positive in order to have a negative TdS value and for a chance for dG to be negative. So why in this problem is the dS stated to be positive?
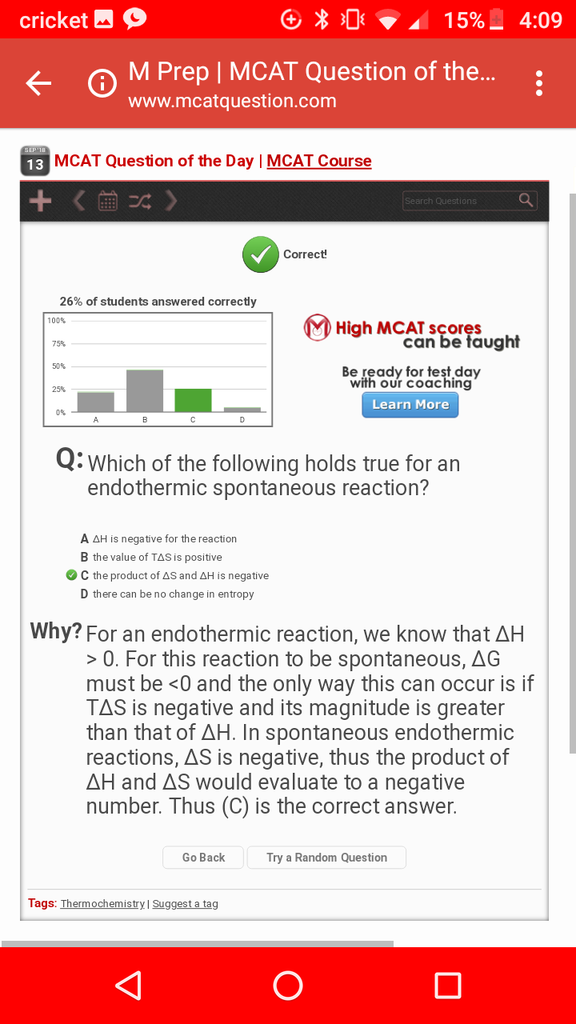
So for endothermic reactions, the dH has to be positive, and if it is spontaneous, the dG has to be negative.
Then dS has to be positive in order to have a negative TdS value and for a chance for dG to be negative. So why in this problem is the dS stated to be positive?
