6
663697
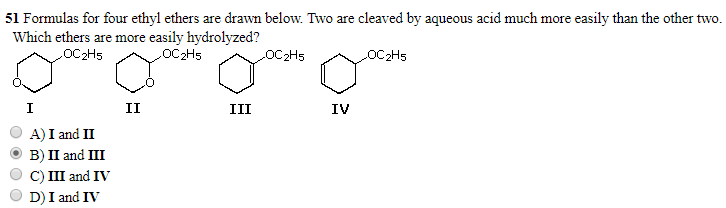
I'm having trouble understanding why II is more easily hydrolyzed than I in the case of acid hydrolysis. Is it because the 2nd electronegative O is closer and pulls more electron density compared to its position in compound I?

I'm having trouble understanding why II is more easily hydrolyzed than I in the case of acid hydrolysis. Is it because the 2nd electronegative O is closer and pulls more electron density compared to its position in compound I?
Just a follow-up question -- when comparing the electrophilicity of 2 different carbonyl carbons, such as between an aldehyde and a ketone, do we only take into account the electron-donating nature if the R groups are alkyl groups? I've only come across a discussion of EDG's reducing the positive nature of the carbonyl carbon of ketones in contrast to aldehydes, so I was wondering if this still applies in cases like acyl halides or carboxylic acids.Yes.
Top of my head, EDGs are usually alkyl. But remember, nitrogen can be ...like NH2.Just a follow-up question -- when comparing the electrophilicity of 2 different carbonyl carbons, such as between an aldehyde and a ketone, do we only take into account the electron-donating nature if the R groups are alkyl groups? I've only come across a discussion of EDG's reducing the positive nature of the carbonyl carbon of ketones in contrast to aldehydes, so I was wondering if this still applies in cases like acyl halides or carboxylic acids.
Just a follow-up question -- when comparing the electrophilicity of 2 different carbonyl carbons, such as between an aldehyde and a ketone, do we only take into account the electron-donating nature if the R groups are alkyl groups? I've only come across a discussion of EDG's reducing the positive nature of the carbonyl carbon of ketones in contrast to aldehydes, so I was wondering if this still applies in cases like acyl halides or carboxylic acids.
