- Joined
- Apr 6, 2010
- Messages
- 2,439
- Reaction score
- 4
- Points
- 4,551
- Medical Student
There is no heat allowed in or out, so q=0.
Free adiabatic expansion is a type of adiabatic expansion where volume stays constant, yeah?
U = Q- W. The internal energy U is related to temperature
Temperature is constant b/c Q = 0 (as pfaction said) AND Work = PΔV= 0
Although why is isothermal expansion a reversible process and free adiabatic expansion an irreversible process?
Free adiabatic expansion is a type of adiabatic expansion where volume stays constant, yeah?
U = Q- W. The internal energy U is related to temperature
Temperature is constant b/c Q = 0 (as pfaction said) AND Work = PΔV= 0
Although why is isothermal expansion a reversible process and free adiabatic expansion an irreversible process?
"Free adiabatic expansion is a type of adiabatic expansion where volume stays constant" That's a fact. Here's a related thread on it:
http://forums.studentdoctor.net/showthread.php?t=834674
Then they start talking about why isothermal is reversible and free adiabatic isn't..and then I lose them. Both EK and Kaplan talk about reversible vs. irreversible process, so I think it's something that may come up.
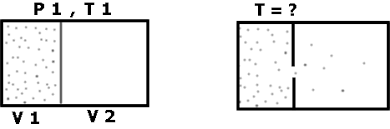
The adiabatic free expansion is an important model transformation for thermodynamics. In a normal adiabatic expansion, such as with an insulated piston, the PV work done by the gas is reflected in a decreased temperature. With the adiabatic free expansion, the final state is equivalent to an isothermal expansion.
This is a 1st law question, but in teaching the adiabatic free expansion is an important model for entropy change where no heat flow occurs. Because the entropy is a state function, you can derive the entropy change in the free expansion using the heat flow from the isothermal transformation which would bring the system from the same initial to final state. However, because the heat flowing into an isothermally expanding system is equaled by heat flowing out of the surroundings, the entropy of the universe doesn't change, even though the entropy of the system has increased. With the adiabatic free expansion, there is no compensating decrease in entropy with the surroundings, so entropy in the universe increases. For this reason, even though the isothermal expansion and the adiabatic free expansion have the same initial and final states, and the same entropy change within the system, the isothermal expansion is reversible while the adiabatic free expansion is not.
"Free adiabatic expansion is a type of adiabatic expansion where volume stays constant" That's a fact. Here's a related thread on it:
http://forums.studentdoctor.net/showthread.php?t=834674
Then they start talking about why isothermal is reversible and free adiabatic isn't..and then I lose them. Both EK and Kaplan talk about reversible vs. irreversible process, so I think it's something that may come up.
Do you have a source that the volume stays constant?"Free adiabatic expansion is a type of adiabatic expansion where volume stays constant" That's a fact. Here's a related thread on it:
http://forums.studentdoctor.net/showthread.php?t=834674
Then they start talking about why isothermal is reversible and free adiabatic isn't..and then I lose them. Both EK and Kaplan talk about reversible vs. irreversible process, so I think it's something that may come up.
Adiabatic free expansion of a gas See also: Free expansion
For an adiabatic free expansion process, the gas is contained in an insulated container and a vacuum. The gas is then allowed to expand in the vacuum. The work done by or on the system is zero, because the volume of the container does not change. The First Law of Thermodynamics then implies that the net internal energy change of the system is zero. For an ideal gas, the temperature remains constant because the internal energy only depends on temperature in that case. Since at constant temperature, the entropy is proportional to the volume, the entropy increases in this case, therefore this process is irreversible
According to these, PV stays constant, but P and V both change. Therefore, V1 is not equal to V2. Where are you reading that volume is constant for an adiabatic free expansion?Free expansion is an irreversible process in which a gas expands into an insulated evacuated chamber.
Real gases experience a temperature change during free expansion. For an ideal gas, the temperature doesn't change, and the conditions before and after adiabatic free expansion satisfy
pi Vi = pf Vf, where p is the pressure, V is the volume, and i and f refer to the initial and final states.
During free expansion, no work is done by the gas. The gas goes through states of no thermodynamic equilibrium before reaching its final state, which implies that one cannot define thermodynamic parameters as values of the gas as a whole. For example, the pressure changes locally from point to point, and the volume occupied by the gas (which is formed of particles) is not a well defined quantity.
The work done by or on the system is zero, because the volume of the container does not change
Ok I think I figured out where all the confusion is coming from in regard to this.
Free adiabatic expansion of an ideal gas means the ideal gas is allowed to expand into a preset volume container, such as the one shown here:

When I initially read this thread, I thought the container was expanding (as in a piston), but that is not the case. The volume of the CONTAINER remains constant, while the volume of the GAS (real or ideal) changes. Sorry if I'm the only one just now realizing this, but it makes things very clear for me so I imagine some of you are encountering the same confusion I was.
The reason it is irreversible is because of entropy. You are not going to get 10 particles to squeeze into a 10mL space if they all have access to a 100mL space unless you put in some energy. Adiabatic = no energy input, so your reaction is not going to reverse to the original state barring being in some universe where entropy is disfavored.
No work is done by the gas because it is simply increasing entropy spontaneously and under no force or energy absorption.
So to answer the OP. Free adiabatic expansion of ideal gas does involve constant temperature. Free adiabatic expansion of real gases will involve a temperature decrease.
It sucks because if the stupid chemists had just termed this "simple effusion of a gas" we would all have gotten it the first time around. But throwing in fancy look-at-how-smart-I-am terminology like "free adiabatic expansion" makes it more complicated than it really is.
yes. to help illustrate (and for me. correct if i'm wrong 😉)

"Irreversible adiabatic process: If the cylinder is a perfect insulator, the initial top-left state cannot be reached anymore after it is changed to the one on the top-right. Instead, the state on the bottom left is assumed when going back to the original pressure because energy is converted into heat."
http://en.wikipedia.org/wiki/Irreve...odynamics)#Examples_of_irreversible_processes
Why is this irreversible?
As medpr said, entropy increases spontaneously. Entropy decreases when energy is put in the system. Isothermal can throw in energy in the system and is therefore reversible. Adiabatic only increases entropy and therefore is irreversible.
Apology accepted, and I probably should have restricted some of my more pointed comments to PM. I will remove them.Yes, you're right. I'm sorry.
