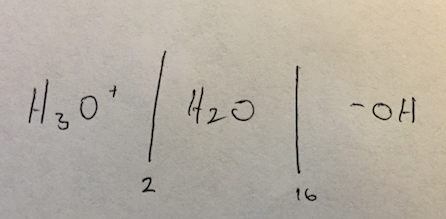- Joined
- Mar 6, 2013
- Messages
- 1,537
- Reaction score
- 2,154
Hey guys. I have come across questions, in destroyer for instance, where a pH / pKA is given and we need to determine which ones would be deprotonated or not. I have looked at the solutions but the concept is still kind of fuzzy. Can anyone explain?