- Joined
- Mar 27, 2011
- Messages
- 147
- Reaction score
- 0
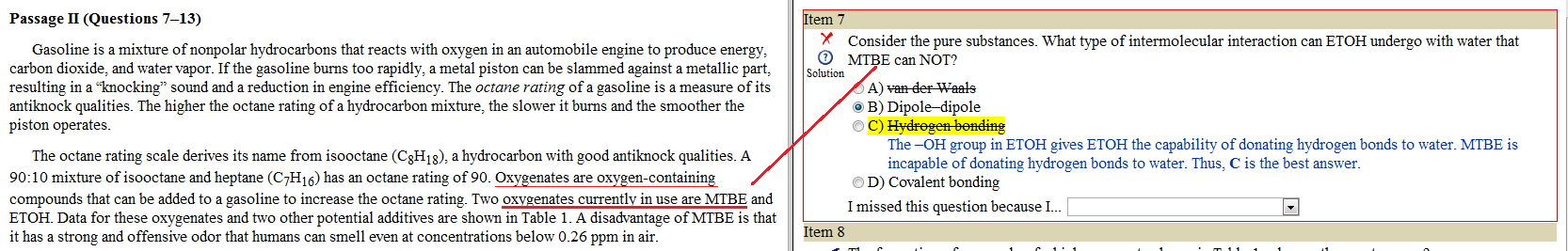
Ok so ethers can H-bond right? Obviously they can't donate a proton for the bond, but in general, if a question asks whether an ether can H-bond, the answer is yes right? (EK said they could).. AAMC9 had a question about MTBE (polar aprotic solvent), and MTBE is an ether so I put yes, but the answer was no. This makes sense because its polar APROTIC, but where do I draw the line???!!!! helppp
basically, how do I know when to put "yes ethers can H-bond" vs "no ethers
cant H-bond"? should I assume ethers can NOT H-bond at all times?
basically, how do I know when to put "yes ethers can H-bond" vs "no ethers
cant H-bond"? should I assume ethers can NOT H-bond at all times?