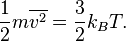Metal #1 has a high heat capacity. Metal #2 has a low heat capacity.
If both metals are at 20 degrees C, their molecules have the same amount of kinetic energy, right?
If both metals are at 20 degrees C, their molecules have the same amount of kinetic energy, right?