Can anyone help explain this to me or provide good links/videos please? I just cant seem to get splitting patterns and stuff down. Thanks!
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
I don't understand 1H NMR
- Thread starter whatwhy
- Start date
- Joined
- May 6, 2010
- Messages
- 2,893
- Reaction score
- 127
Have you looked at Khan Academy? He explains the method pretty well.
It's also one of those concepts that you need to practice. EK orgo 1001 has a ton of those practice problems.
It's also one of those concepts that you need to practice. EK orgo 1001 has a ton of those practice problems.
- Joined
- Jun 29, 2011
- Messages
- 1,721
- Reaction score
- 293
Also a topic that you can most likely get away with not knowing the concepts and just being able to do the related problems.. because there's a bunch of very common questions that aren't too difficult to learn how to do.
You don't really need to be an H NMR buff to get MCAT-level questions correct.
You don't really need to be an H NMR buff to get MCAT-level questions correct.
Thanks guys. Here's a question: for CH3CHCL2, there's a quartet
 .
.
For trichloroethane theres a triplet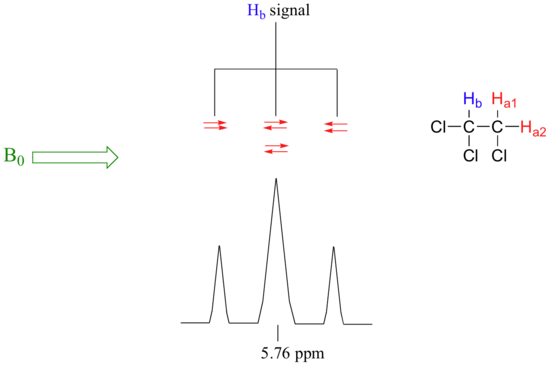 .
.
How come for the first example there are two peaks in the middle, basically two columns with opposite spins, and for the second example theres just one peak in the middle with only one column. Hell, why aren't all those in one column? I understand the n+1 rule but it makes no sense to me that for example 1 they can have those opposite spins in two columns but for example 2 they can have all those opposite spins in one.
Thanks...hopefully this makes some sense.

For trichloroethane theres a triplet
 .
. How come for the first example there are two peaks in the middle, basically two columns with opposite spins, and for the second example theres just one peak in the middle with only one column. Hell, why aren't all those in one column? I understand the n+1 rule but it makes no sense to me that for example 1 they can have those opposite spins in two columns but for example 2 they can have all those opposite spins in one.
Thanks...hopefully this makes some sense.
- Joined
- Jun 23, 2013
- Messages
- 958
- Reaction score
- 413
First thing you want to look for is unique hydrogen environments (be careful here).Thanks guys. Here's a question: for CH3CHCL2, there's a quartet.
For trichloroethane theres a triplet.
How come for the first example there are two peaks in the middle, basically two columns with opposite spins, and for the second example theres just one peak in the middle with only one column. Hell, why aren't all those in one column? I understand the n+1 rule but it makes no sense to me that for example 1 they can have those opposite spins in two columns but for example 2 they can have all those opposite spins in one.
Thanks...hopefully this makes some sense.
In your fist example, there are two hydrogen environments, so we expect to see two signals.
We can also establish the relative area under each signal (1:3).
To determine the splitting pattern due to the presence of neighboring hydrogens, follow the n+1 rule:
The solo hydrogen has 3 hydrogen neighbors, so according to n+1 (ie. 3+1), we expect 4 peaks (a quartet). Likewise, the methyl hydrogens experience doublet splitting (2 peaks), due to presence of the solo neighboring hydrogen.
---
In the second example, there is yet again, two hydrogen environments -- therefore 2 signals.
The ratio of the signals are 1:2 (area under the signal).
To determine the splitting pattern in the second example, the solo hydrogen has two neighbors. Therefore, according to n+1 coupling/splitting rule, we expect a triplet for that signal (because 2+1 = 3). Likewise, the two hydrogens next to the solo hydrogen experience a doublet (2 peaks), again due to the presence of single neighboring hydrogen (n+1 --> 1+1=2).
---
The first picture has to do with spin states -- how the neighboring methyl hydrogens effect the splitting of the solo hydrogen group. The protons of the 3 hydrogens on the methyl group can all spin with the magnetic field, against the magnetic field, or something in between. This is why we expect the neighboring hydrogen to have 4 peaks.
The second picture is considering the effect the two neighboring hydrogens on the carbon with a signal hydrogen. For the same reasons explained above (because the proton spin states can orient themselves in different ways), this is essentially why we expect 3 peaks for that hydrogen.
Those pictures explain the WHY we see what we see, but honestly, just knowing n+1 will help you answer the question lol.
- Joined
- Jun 29, 2011
- Messages
- 1,721
- Reaction score
- 293
The splitting (i.e. the number of peaks) is determined by the # of chemically different neighboring protons, as you referred to. The quartet in CH3CHCL2 is for the bolded hyrodgen.. which has 3 neighboring protons so 4 total peaks. 4 peaks = quartet.
For trichloroethane, Hb in the picture is split by two neighboring protons (Ha's).. so 3 total peaks = triplet.
How the peaks/splitting are derived is, frankly, probably a little out of scope for the MCAT but it has to do with spin of the neighboring proton and how they shield/deshield the proton in question. If they align (up) with the spectrometer field, it will shield.. if they are opposite (down), it will deshield. This will affect WHERE that peak shows up.. which leads to what we know as peak splitting (a little bit off where we expect if no splitting occurred so they're all grouped together still).
For CH3CHCL2, there are 3 total protons, each with their own spin. So let's think through the combinations:
Up Up Up
Up Up Down
Up Down Up
Up Down Down
Down Up Up
Down Up Down
Down Down Up
Down Down Down
We have to think of the net effect of these spins. 3 "up"s is different than 3 "down"s. So we basically have four categories:
3 Ups
2 Ups, 1 Down
2 Downs, 1 Up
3 Downs
Each of these affects where the peak falls out so each of these will be its own peak.
The intensity (peak height) is determined by the 'members' of each group:
3 Ups has 1 possible combination
2 Ups, 1 Down has 3 possible combinations
2 Downs, 1 Up has 3 possible combinations
3 Downs has 1 possible combination
leading us to 4 peaks (quartet) of a 1:3:3:1 height ratio.
For trichloroethane (think through this yourself before continuing):
2 protons so combinations are:
Up Up
Up Down
Down Up
Down Down
1- 2 Ups
2- 1 Up, 1 Down
1- 2 Downs
so 3 peaks with a 1:2:1 height ratio.
For trichloroethane, Hb in the picture is split by two neighboring protons (Ha's).. so 3 total peaks = triplet.
How the peaks/splitting are derived is, frankly, probably a little out of scope for the MCAT but it has to do with spin of the neighboring proton and how they shield/deshield the proton in question. If they align (up) with the spectrometer field, it will shield.. if they are opposite (down), it will deshield. This will affect WHERE that peak shows up.. which leads to what we know as peak splitting (a little bit off where we expect if no splitting occurred so they're all grouped together still).
For CH3CHCL2, there are 3 total protons, each with their own spin. So let's think through the combinations:
Up Up Up
Up Up Down
Up Down Up
Up Down Down
Down Up Up
Down Up Down
Down Down Up
Down Down Down
We have to think of the net effect of these spins. 3 "up"s is different than 3 "down"s. So we basically have four categories:
3 Ups
2 Ups, 1 Down
2 Downs, 1 Up
3 Downs
Each of these affects where the peak falls out so each of these will be its own peak.
The intensity (peak height) is determined by the 'members' of each group:
3 Ups has 1 possible combination
2 Ups, 1 Down has 3 possible combinations
2 Downs, 1 Up has 3 possible combinations
3 Downs has 1 possible combination
leading us to 4 peaks (quartet) of a 1:3:3:1 height ratio.
For trichloroethane (think through this yourself before continuing):
2 protons so combinations are:
Up Up
Up Down
Down Up
Down Down
1- 2 Ups
2- 1 Up, 1 Down
1- 2 Downs
so 3 peaks with a 1:2:1 height ratio.
- Joined
- May 6, 2010
- Messages
- 2,893
- Reaction score
- 127
lolwut
- Joined
- Jun 29, 2011
- Messages
- 1,721
- Reaction score
- 293
I think this thread tells me that I've forgotten all of my orgo...
The concepts behind NMR are out of scope on the MCAT. If you're asked it, you're likely to get background information in a passage.
Just know how to work with splitting to identify which proton is being talked about, etc.
- Joined
- Jul 22, 2011
- Messages
- 2,036
- Reaction score
- 990
The concepts behind NMR are out of scope on the MCAT. If you're asked it, you're likely to get background information in a passage.
Just know how to work with splitting to identify which proton is being talked about, etc.
Lol I was just commenting on how quickly we forget things after taking the MCAT and applying to med school.
- Joined
- Jun 29, 2011
- Messages
- 1,721
- Reaction score
- 293
Lol I was just commenting on how quickly we forget things after taking the MCAT and applying to med school.
yea, things like this become pretty irrelevant also. I only remember NMR because my Orgo professor drilled it into our heads.
- Joined
- Jun 23, 2013
- Messages
- 958
- Reaction score
- 413
Yeah, I'll be dumping most of my Physics knowledge in the trashbin to make room for other med school topics. It's actually kinda daunting -- after seeing what my cousin (whose in med school) has to study on a weekly basis. His counters and floors are piled high with notes and he literally lives and breathes studying. It makes the MCAT seem like a joke, but right now it's a big hurdle I need to overcome to reach my goal.Lol I was just commenting on how quickly we forget things after taking the MCAT and applying to med school.
- Joined
- Jul 22, 2011
- Messages
- 2,036
- Reaction score
- 990
Yeah, I'll be dumping most of my Physics knowledge in the trashbin to make room for other med school topics. It's actually kinda daunting -- after seeing what my cousin (whose in med school) has to study on a weekly basis. His counters and floors are piled high with notes and he literally lives and breathes studying. It makes the MCAT seem like a joke, but right now it's a big hurdle I need to overcome to reach my goal.
Well I'll be looking forward to that in 7 months time lol. Good luck to you! This test is brutal and the Prometric setup doesn't make it easier either. It's almost like a jail cell in there. They have cameras literally everywhere watching your every move.
- Joined
- Jun 23, 2013
- Messages
- 958
- Reaction score
- 413
That's out of scope imo. You'd be fine just to know (n+1). I'm not sure why TBR feels the need to emphasize that.Learn pascal's triangle to understand the pattern!
- Joined
- Jun 29, 2011
- Messages
- 1,721
- Reaction score
- 293
That's out of scope imo. You'd be fine just to know (n+1). I'm not sure why TBR feels the need to emphasize that.
Eh.. I think that's a fair enough question. Also fairly easy to prepare for so worth the investment. Don't memorize the triangle, just know how to produce it.
That doesn't explain why though
- Joined
- Jan 17, 2013
- Messages
- 1,902
- Reaction score
- 1,538
I don't mean to memorize it, just knowing the structure can intuitively bring about the image of coupling in your head in an easy way. The n+1 is the golden rule for sure.That's out of scope imo. You'd be fine just to know (n+1). I'm not sure why TBR feels the need to emphasize that.
Similar threads
- Replies
- 5
- Views
- 3K
D
- Replies
- 7
- Views
- 1K
D
