- Joined
- Feb 13, 2014
- Messages
- 507
- Reaction score
- 322
Hi fellow pre-dents,
I have a question regarding intramolecular aldol condensation. In the following video for the first example, I am confused as to how to know that we would take the alpha H off of carbon 1 versus C3. I understand that the resulting product has too much strain, but on the DAT is there an easy way to figure this out without having to draw out the structures of both the C1 and C3 products? I included a picture as well in case you don't feel like watching the first few minutes of the video. Thanks!
https://www.khanacademy.org/science...ation-jay/v/intramolecular-aldol-condensation
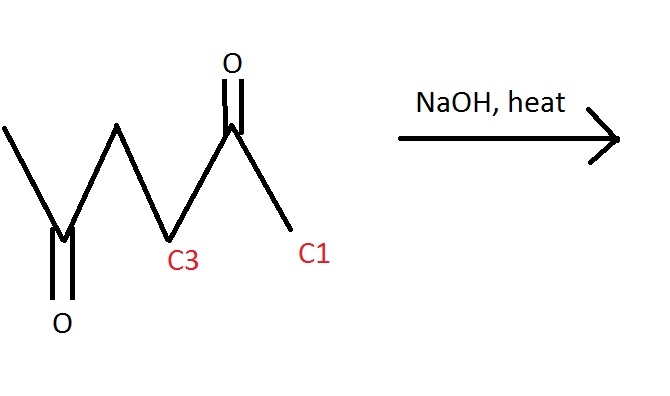
(How do you know to steal the H from C1 versus C3?)
EDIT: I may have answered my own question. I was thinking C3's H was more acidic because it's in between two carbonyls, but it's actually the end H that is more acidic and thus will react, correct? This leads me to a second question... does anyone have any good videos or advice on finding the most acidic proton? I seriously suck at this!
I have a question regarding intramolecular aldol condensation. In the following video for the first example, I am confused as to how to know that we would take the alpha H off of carbon 1 versus C3. I understand that the resulting product has too much strain, but on the DAT is there an easy way to figure this out without having to draw out the structures of both the C1 and C3 products? I included a picture as well in case you don't feel like watching the first few minutes of the video. Thanks!
https://www.khanacademy.org/science...ation-jay/v/intramolecular-aldol-condensation
(How do you know to steal the H from C1 versus C3?)
EDIT: I may have answered my own question. I was thinking C3's H was more acidic because it's in between two carbonyls, but it's actually the end H that is more acidic and thus will react, correct? This leads me to a second question... does anyone have any good videos or advice on finding the most acidic proton? I seriously suck at this!
Last edited:
