- Joined
- Feb 16, 2016
- Messages
- 594
- Reaction score
- 96
According to the following standard reduction potentials
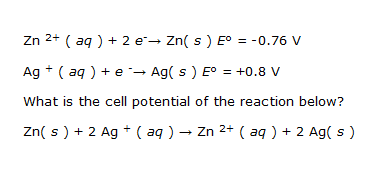
So I always get confused as to why they switched the sign for the standard reduction potential for Zn from -0.76 to +0.76.
The way I look at it, from the reaction given, Zn is oxidized and Ag is reduced. As for the standard reduction potentials table given, it shows that the more + the standard reduction potential is, the more likely it is for that species to be reduced. Table shows that Ag is +0.8 and Zn is -0.76, so therefore, Ag is the species that is likely to be reduced while the less positive one gets oxidized, the Zn. So from the reaction, it shows exactly that - Ag is reduced and Zn is oxidized, so why the need to reverse only the sign for Zn?
I have always encountered these problems where sometimes they would switch the signs, sometimes they won't. Can someone who legit understands this help me out? This really irks me cause this always seems to happen. Thanks again!
So I always get confused as to why they switched the sign for the standard reduction potential for Zn from -0.76 to +0.76.
The way I look at it, from the reaction given, Zn is oxidized and Ag is reduced. As for the standard reduction potentials table given, it shows that the more + the standard reduction potential is, the more likely it is for that species to be reduced. Table shows that Ag is +0.8 and Zn is -0.76, so therefore, Ag is the species that is likely to be reduced while the less positive one gets oxidized, the Zn. So from the reaction, it shows exactly that - Ag is reduced and Zn is oxidized, so why the need to reverse only the sign for Zn?
I have always encountered these problems where sometimes they would switch the signs, sometimes they won't. Can someone who legit understands this help me out? This really irks me cause this always seems to happen. Thanks again!
