Where is the free hemiacetal or hemiketal in maltose?

Is it the hemiaketal with the central carbon being carbon no5?
Is it the hemiaketal with the central carbon being carbon no5?
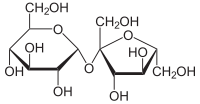

B/c of enediol rearrangement. The ketose actually converts back and forth into an aldose.TPR says ketones react with Benedict's.


