- Joined
- Nov 10, 2009
- Messages
- 138
- Reaction score
- 0
Methyl Benzoate:
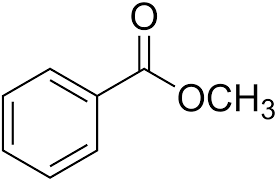
According to Kaplan, this NMR spectrum is for methyl benzoate:

I dont understand this. From the structure of methyl benzoate, there should be four groups:
1) hydrogen at para (int = 1)
2) hydrogens a bit closer to ester on benzene ring (int = 2)
3) hydrogens again, a bit closer to the ester on benzene ring (int = 2)
4) hydrogens on the methyl group (int 3)
However, the number of peaks and the integration values on the NMR are 3, 3, 2... Is this right?
Thanks for you help!
According to Kaplan, this NMR spectrum is for methyl benzoate:

I dont understand this. From the structure of methyl benzoate, there should be four groups:
1) hydrogen at para (int = 1)
2) hydrogens a bit closer to ester on benzene ring (int = 2)
3) hydrogens again, a bit closer to the ester on benzene ring (int = 2)
4) hydrogens on the methyl group (int 3)
However, the number of peaks and the integration values on the NMR are 3, 3, 2... Is this right?
Thanks for you help!
