I have read that Guanine has 2 hydrogen bond donors (and 1 acceptor), as shown below. My question is: why is N9 (#9 - NH) not also considered a hydrogen donor, and why is N7 (#7) and N3 (#3) not considered hydrogen acceptors, as these will also have lone pairs?
So shouldn't Guanine have 3 donors, and 3 acceptors? I see that only the left side of the molecule is used for interaction, so is the reasoning that only 2 donors, and 1 acceptor because the "right" side of this molecule is attached to the phosphate backbone, while the "left" side participates in hydrogen bonding?
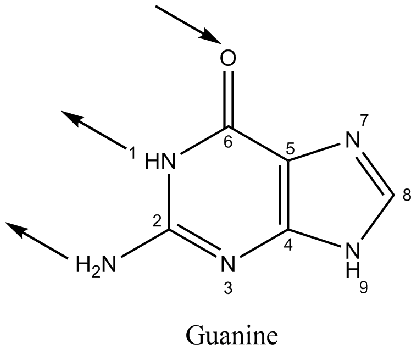
So shouldn't Guanine have 3 donors, and 3 acceptors? I see that only the left side of the molecule is used for interaction, so is the reasoning that only 2 donors, and 1 acceptor because the "right" side of this molecule is attached to the phosphate backbone, while the "left" side participates in hydrogen bonding?
