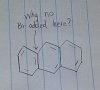
1. For question 2, why cant you add the bromine to the double bond indicated in the picture?
2. For question 7, the answer says that the compounds in the question can be isolated by crystallization, and the para-isomer will crystallize out of solution, but the ortho isomer will not.
How can you tell which one precipitate out based on the given structure?
3. For question 24, why being more branched give the lowest heat of combustion?
2. For question 7, the answer says that the compounds in the question can be isolated by crystallization, and the para-isomer will crystallize out of solution, but the ortho isomer will not.
How can you tell which one precipitate out based on the given structure?
3. For question 24, why being more branched give the lowest heat of combustion?