Hi,
I came across this question today about calculating the net charge on a peptide at pH 7, and I don't get where I'm going wrong.
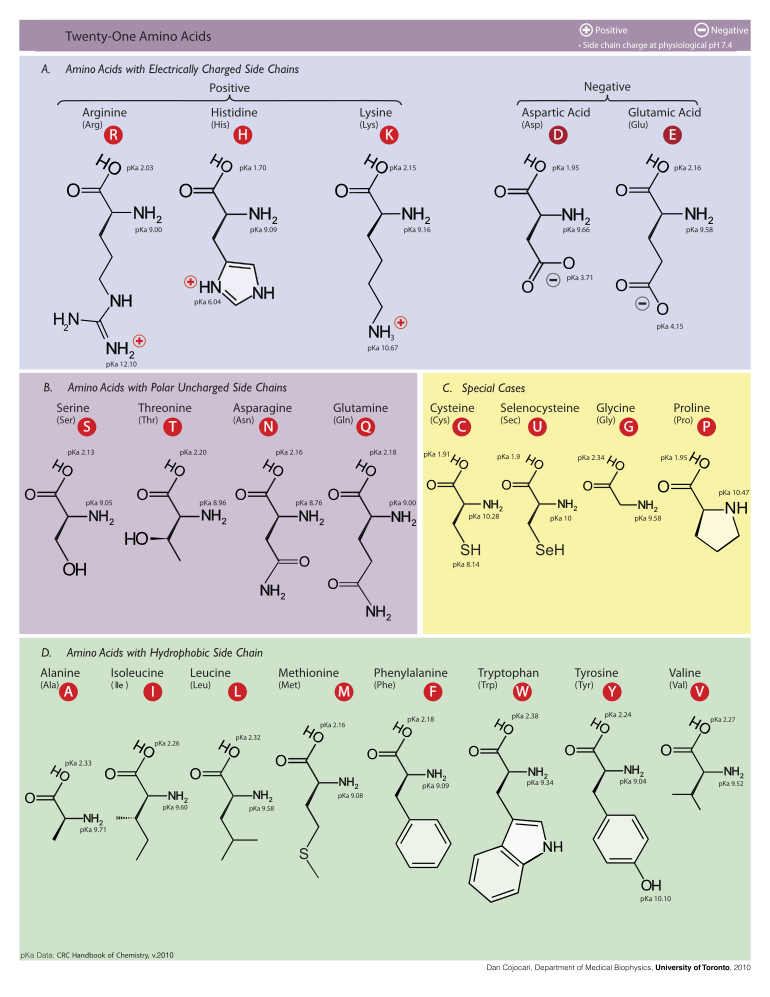
The peptides given were:
A) AVDEKMSTRGHKNPG
B) YPGRSMHEWDIKAQP
C) HIPAGEATEKALRGD
D) EAPDTSEGDLIPEUS
According the answer, only C and D have a negative net charge. C has a negative net charge of -1, while D has a net charge of -5. Using the rule that basic amino acids (histidine, lysine, and arginine) provide a +1 charge, while acidic amino acids (glutamate and aspartate) provide a -1 charge, I thought the answer would be:
A) -2
B) -1
C) 0
D) -5
What am I missing here??
Thank you!
I came across this question today about calculating the net charge on a peptide at pH 7, and I don't get where I'm going wrong.
The peptides given were:
A) AVDEKMSTRGHKNPG
B) YPGRSMHEWDIKAQP
C) HIPAGEATEKALRGD
D) EAPDTSEGDLIPEUS
According the answer, only C and D have a negative net charge. C has a negative net charge of -1, while D has a net charge of -5. Using the rule that basic amino acids (histidine, lysine, and arginine) provide a +1 charge, while acidic amino acids (glutamate and aspartate) provide a -1 charge, I thought the answer would be:
A) -2
B) -1
C) 0
D) -5
What am I missing here??
Thank you!