- Joined
- Dec 17, 2007
- Messages
- 3,390
- Reaction score
- 4,403
Hey, I wanna hear your feedback on this one.
I just found out that this trial is running
 protecttrial.eu
protecttrial.eu
A randomized Phase III trial on photons vs. protons for neoadjuvant chemorads in esophageal cancer.
The trial will cost roughly 5 million dollars

 www.imi.europa.eu
www.imi.europa.eu
The primary endpoint of the trial is pneumonitis >G1 and the trial is powered to detect a 10% difference.
 protecttrial.eu
protecttrial.eu
Bearing in mind that pneumonitis was not a major issue in the completed, randomized phase II trial from the US
(Randomized Phase IIB Trial of Proton Beam Therapy Versus Intensity-Modulated Radiation Therapy for Locally Advanced Esophageal Cancer - PubMed), I wonder if the Phase III trial is worth the 5 millions spent?
Here are the pneumonitis events in the US trial
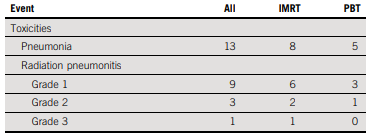
Accrual was 72 patients with IMRT, 73 with protons
And frankly, even if it comes out positive and there's a 12% difference in pneumonitis, but it's mainly grade 2 pneumonitis...

Thoughts?
I just found out that this trial is running
protecttrial.eu
A randomized Phase III trial on photons vs. protons for neoadjuvant chemorads in esophageal cancer.
The trial will cost roughly 5 million dollars

IMI Innovative Medicines Initiative | PROTECT-trial | Proton versus photon therapy for esophageal cancer - a trimodality strategy
Radiotherapy plays a key role in the treatment of many cancers. However, as conventional radiotherapy can cause side effects in surrounding organs, the dose is limited meaning that treatment takes longer and can be less effective. Proton therapy is an innovative form of radiotherapy. As the...
The primary endpoint of the trial is pneumonitis >G1 and the trial is powered to detect a 10% difference.
Protocol
Bearing in mind that pneumonitis was not a major issue in the completed, randomized phase II trial from the US
(Randomized Phase IIB Trial of Proton Beam Therapy Versus Intensity-Modulated Radiation Therapy for Locally Advanced Esophageal Cancer - PubMed), I wonder if the Phase III trial is worth the 5 millions spent?
Here are the pneumonitis events in the US trial
Accrual was 72 patients with IMRT, 73 with protons
And frankly, even if it comes out positive and there's a 12% difference in pneumonitis, but it's mainly grade 2 pneumonitis...

Thoughts?
Last edited:

