- Joined
- Jun 2, 2010
- Messages
- 295
- Reaction score
- 2
Hey all,
I'm not sure about this two reactions in the picture I drew. they are part of the reaction mechanisms in question 77 and 78 in the destroyer.
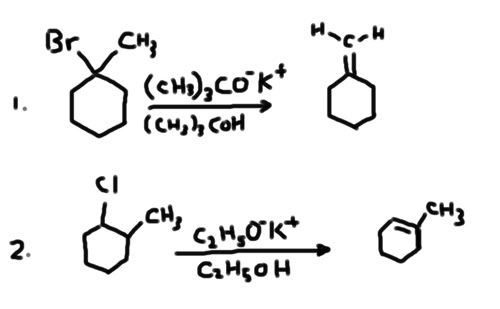
Can someone please tell me what's the name of this type of reaction so I can google it? I was thinking it's a E1 or E2. However, it can't be E2 because the base is not strong enough. It shouldn't be E1 either because E1 and Sn1 always compete and it's hard to make a reaction just do E1. So I'm really stuck and would like some help.
In general, you have a halide, and you react it with RO-K+/ROH, what's the name of this reaction?
Thanks 🙂
I'm not sure about this two reactions in the picture I drew. they are part of the reaction mechanisms in question 77 and 78 in the destroyer.

Can someone please tell me what's the name of this type of reaction so I can google it? I was thinking it's a E1 or E2. However, it can't be E2 because the base is not strong enough. It shouldn't be E1 either because E1 and Sn1 always compete and it's hard to make a reaction just do E1. So I'm really stuck and would like some help.
In general, you have a halide, and you react it with RO-K+/ROH, what's the name of this reaction?
Thanks 🙂
