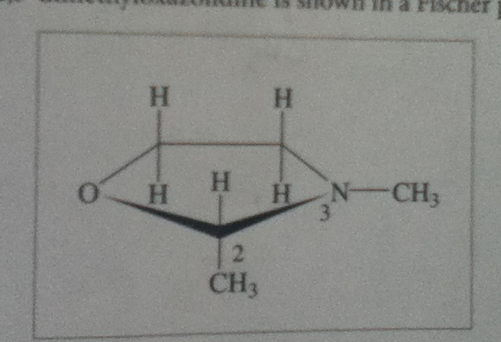
How many types of protons will most likely be dected by high-resolution 1H-NMR at room temperature? Answer: 7
How many types of protons will be detected when the H on the stereogenic carbon is replaced with an methyl group? Answer : 4
I'm surprised that suddenly the two H of the CH2 groups are equivalent?

