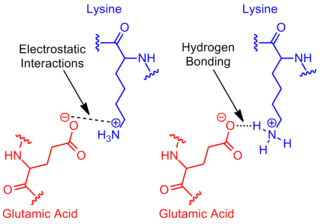
i was doing a practice question that asked what would happen if you change isoleucine to lysine in a protein where the isoleucine was located close to Glu. According to the answer, it says that this change will make it more stable (which i get) but it then goes on to say that because it is more stable is is more likely to give up its proton (making it more acidic). if something is more stable, then wouldnt it be less likely to give up its proton? basically why would it be more acidic? isnt lysine a basic amino acid?
question about amino acids
- Thread starter morrisol
- Start date
Similar threads
- Question