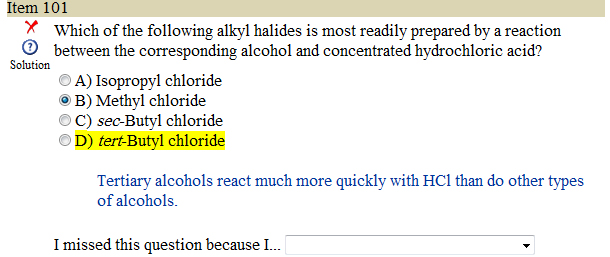- Joined
- Jun 29, 2004
- Messages
- 203
- Reaction score
- 2
Please help !!
Looking at the reactions below which product is most readily prepared and why ?

Thanks in advance
Looking at the reactions below which product is most readily prepared and why ?

Thanks in advance