- Joined
- May 30, 2020
- Messages
- 117
- Reaction score
- 52
What is the total number of isomers with the formula C3H6O that are either cyclic or chiral?
The answer was 6 isomers on Khan Academy, and the hints are provided below. But I don't seem to understand why the cis/trans non cyclic isomers can be considered chiral. I mean is there an explanation I am missing?
Hint #11 / 4
Let’s consider possible cyclic compounds. The oxygen will be single bonded either as an ether and part of the ring structure or as an alcohol and outside any ring structure. Whether the nomenclature of the following structures is apparent or not is not important, but there are three possible structural isomers: cyclopropanol, oxetane, and methyl oxirane.

Hint #22 / 4
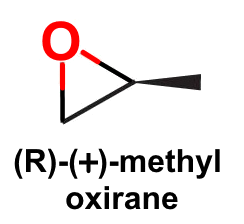
Two isomers, propen-1-ol and methyl oxirane, have a stereogenic center, but from methyl oxirane there will be a set of enantiomers:

Hint #33 / 4
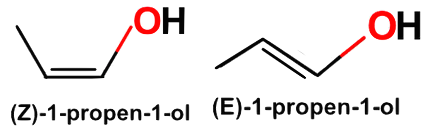
Propen-1-ol’s geometric isomers are diastereomeric, and the count for stereoisomers is four:
Hint #44 / 4
Since there is overlap of methyl oxirane in both categories, the total number of isomers is 6.
The answer was 6 isomers on Khan Academy, and the hints are provided below. But I don't seem to understand why the cis/trans non cyclic isomers can be considered chiral. I mean is there an explanation I am missing?
Hint #11 / 4
Let’s consider possible cyclic compounds. The oxygen will be single bonded either as an ether and part of the ring structure or as an alcohol and outside any ring structure. Whether the nomenclature of the following structures is apparent or not is not important, but there are three possible structural isomers: cyclopropanol, oxetane, and methyl oxirane.

Hint #22 / 4
Two isomers, propen-1-ol and methyl oxirane, have a stereogenic center, but from methyl oxirane there will be a set of enantiomers:


Hint #33 / 4
Propen-1-ol’s geometric isomers are diastereomeric, and the count for stereoisomers is four:

Hint #44 / 4
Since there is overlap of methyl oxirane in both categories, the total number of isomers is 6.
