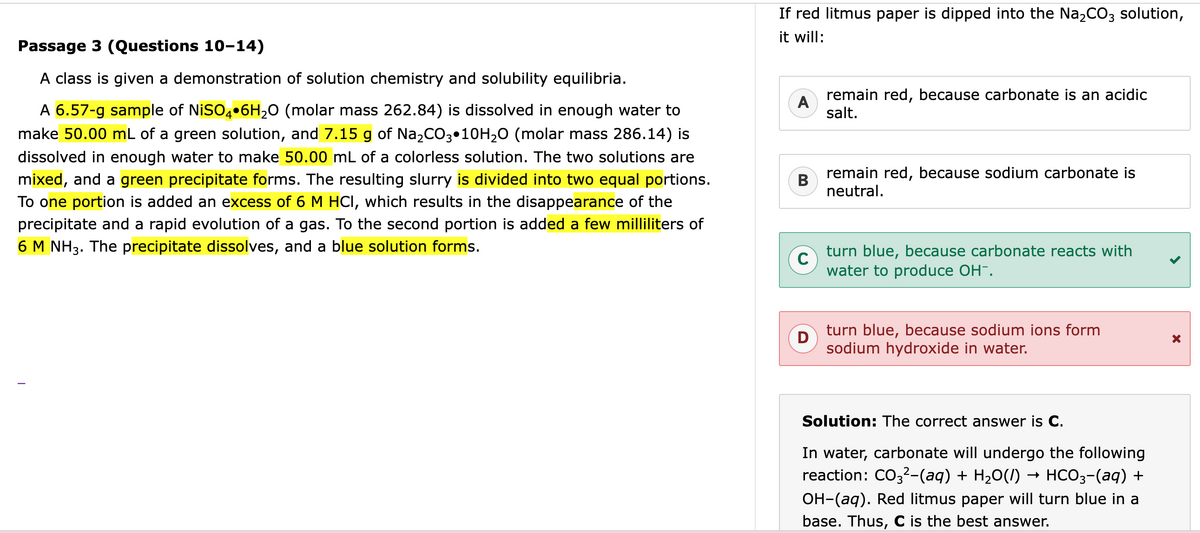
You
don't have Na (the metal) in the mixture, you have Na+ ions from the ionic solution of Na2CO3. That eliminates D. Na metal is what would react with water to form OH- ions in a (very vigorous) redox reaction. Na+ ions have already given that one valence electron away and so are very happy living as ions in the polar solvent of water.
As to why the answer is C, carbonate is a weak acid and hence a strong base (i.e. reacts with water to form OH- ions). That is probably something you can be assumed to know based on acid-base chemistry. In particular, I have a hunch they ask about carbonate here
specifically because it is involved in respiration/physiological pH regulation. Look at the
Khan Academy page for it, maybe it will help.