Is it true that the larger R groups prefer to be equatorial and the smaller groups prefer to be axial? Is this always true, say, for any cyclohexane that has two R groups on opposite sides?
Yes, bulky R groups definitely want to avoid axial positions if at all possible because substituents in the axial position experience what is often referred to as "1,3-diaxial interactions," which essentially is a fancy term referring to steric hinderance associated with substituents in this position.
Whenever you encounter substituted cyclohexanes, immediately consider the chair conformation. Substituents WANT to be in the equatorial position. So... if cyclohexane had just one R group attached to the ring, then by default it would favor the equatorial position because ultimately, this is the more stable form.
However, you'll likely encounter a situation with 2 or more substituents attached to the ring, in which case you really have to analyze things more carefully, because depending on how substituents are oriented about the ring, they all may or may not be (able to be) in the equatorial position. Instead, you may face a situation in which SOME substituents can be placed equatorially, while others must be placed in the axial position (again, due to their orientation). In this scenario, because you have to pick and choose, you'd want the bulkier substituent to be the equatorial position to reduce the unfavorable interactions described earlier. So for example, if you had a situation where you had to choose between a bulky t-Butyl group, or a methyl ... obviously you'd want the t-Butyl group to be in the equatorial position (or even better, both if it were physically possible).
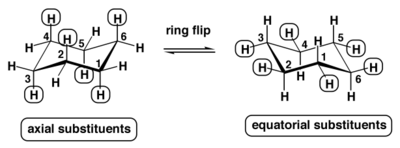
Now to understand why it may not always be possible to put both substituents in the equatorial position, you need a decent understanding of both chair conformations (ring flip):
Notice, according to the picture, all axial positions switch to equatorial during a ring flip (blue hydrogens).
Also notice that anything pointing UP remains UP, regardless if they are axial or equatorial. Likewise, anything pointing DOWN, remains DOWN.
So what does this mean?
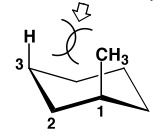
Consider this example (look at image II)
Here's how I would solve this problem:
#1 -First, I realize I have two substituents: a methyl group and a t-butyl group.
#2 - Immediately, you realize that tButyl is the bulkier group, so let's assume it's already in the equatorial position. (Let's say: tButyl Wedge=Equatorial).
Great! Now we have to determine whether the methyl group will also be equatorial or be axial.
Here's a really helpful tip (you might want to refer to the colored picture above). If WEDGE is equatorial for one carbon (let's say tButyl) then the DASH for the neighboring carbon will be equatorial ... this carbons neighbor will have the equatorial position as a WEDGE... and so on. Notice the pattern? It may take a while to understand this concept (took me long enough), but once you realize this, solving these problems are a breeze.
So, if we take tButyl (which is a wedge in that picture) -- and because by default we put it in the equatorial position (due to favored stability), we then know that (according to the pattern above), for it's neighboring carbon , DASH = EQUATORIAL. The next carbon over, WEDGE = EQUATORIAL. Then we finally reach the methyl group. Here we find that DASH = EQUATORIAL. But where is the methyl group pointing? A WEDGE? ....yikes. So because it's not a dash (which would indicate it's equatorial), we know that it MUST be axial.
Hopefully this makes sense
😛