D
deleted388502
Hi guys,
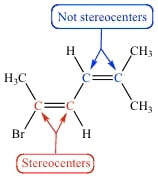
Just wanted to clarify the difference between chiral centers and stereo centers?
I was under the impression that double bond carbons were stereo centers, but I just did an ochem passage that suggested otherwise so I wanted to clear it up.
Thanks!!
Just wanted to clarify the difference between chiral centers and stereo centers?
I was under the impression that double bond carbons were stereo centers, but I just did an ochem passage that suggested otherwise so I wanted to clear it up.
Thanks!!