- Joined
- Dec 2, 2015
- Messages
- 438
- Reaction score
- 23
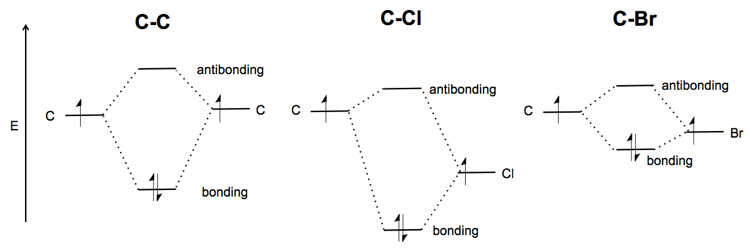
what determines the strength of covalent bonds that lead to bond energy values?
In the equation delta H = BE broken -BE formed, is the bond being broken heterolytic or homolytic?
In the equation delta H = BE broken -BE formed, is the bond being broken heterolytic or homolytic?