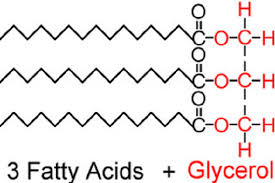
The chemical structures shown in the passage are a triglyceride (Figure 1), phosphatidylcholine (Figure 2), cholesterol (Figure 3), and arachidonic acid (Figure 4). The arrangement of these lipids in order of decreasing polarity is:
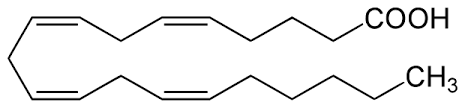
Arachidonic acid
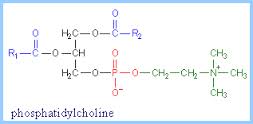
Correct Answer: A) phosphatidylcholine>arachidonic acid>a triglyceride>cholesterol
The explanation in the back first says that the molecule that has a full charge or charges is the most polar, which would be phosphatidylcholine. Then, it says that O is very electronegative, so the molecule with the more O oxygen atoms would be more polar. But it says that triglyceride and arachidonic acid have two oxygen atoms and that is not the case.
Arachidonic acid
Correct Answer: A) phosphatidylcholine>arachidonic acid>a triglyceride>cholesterol
The explanation in the back first says that the molecule that has a full charge or charges is the most polar, which would be phosphatidylcholine. Then, it says that O is very electronegative, so the molecule with the more O oxygen atoms would be more polar. But it says that triglyceride and arachidonic acid have two oxygen atoms and that is not the case.
