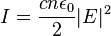I was wondering is there any relation to waves, frequency, and intensity. If you increase the frequency of a wave does that increase the intensity of the wave? What is the relation between wavelength and intensity. Also how do these concepts apply to particles. Is the relation E=(frequency)(wavelength) for particles, correct?
Just want to add one thing here... don't know how helpful this will be, but when you mentioned intensity/freq, what immediately came to mind was the photoelectric effect.
For the photoelectric effect, the KE of the ejected electron is dependent solely on the *frequency* of the incident radiation. But, the # of electrons ejected has to do with both the frequency and the *intensity* of the incident light. Specifically, higher intensity radiation will kick off more electrons. But the KE each of those electrons has has to do with the frequency of the radiation, not the intensity.
There's a cutoff frequency below which, light will not eject any electrons from the metal. This is why red light (low freq) of any intensity will not eject any electrons-- it doesn't have enough energy (as dictated by its freq). But past that cutoff frequency, the # of electrons that get ejected has to do with the light's intensity. Anyway, I think it's more helpful to understand the distinctions between concepts like that in terms of physical phenomena, instead of just thinking about them in terms of equations...
I think of intensity as like "brightness" (for light) or "volume" (for sound). Frequency for light is color, if we're talking visible spectrum, or different types of radiation (IR vs. x-ray), if we're talking about the whole spectrum. Frequency for sound is like pitch. So, if you think about it that way, it's clear that "brightness" and color are really distinct concepts, for intensity and frequency are really distinct concepts too. Somebody correct me if I'm wrong.