You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
What is this reaction called? What is going on?
- Thread starter 510586
- Start date
- Joined
- Mar 1, 2015
- Messages
- 65
- Reaction score
- 27
Correct me if I'm wrong, but this is a sn2 mechanism thats involves a tosylate.
So like Williamson kind of? The end result looks like itCorrect me if I'm wrong, but this is a sn2 mechanism thats involves a tosylate.
- Joined
- Mar 12, 2005
- Messages
- 5,624
- Reaction score
- 3,534
View attachment 193553 Would this be some kind of Williamson ether synthesis? No idea
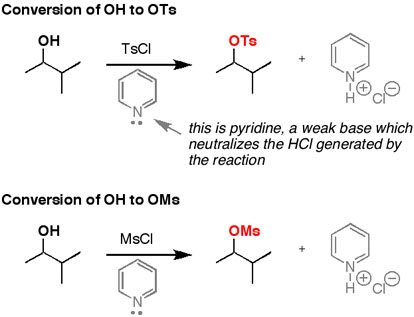
This reaction is the formation of a sulfonate ester. We do this to create a good leaving for a nucleophilic attack. Recall the OH group is not a good leaving group under most circumstances.
This is a substitution type of a mechanism ...think of it as SN2-like.
Hope this helps.
Dr. Romano
- Joined
- Feb 9, 2014
- Messages
- 77
- Reaction score
- 17
in sn2 with tosylate, shouldn't the tosylate leave? It's like the best leaving group in the game...
It is a SN2 reaction where the Cl- leaves.
Oh so is this just like the O Ts reactions?This reaction is the formation of a sulfonate ester. We do this to create a good leaving for a nucleophilic attack. Recall the OH group is not a good leaving group under most circumstances.
This is a substitution type of a mechanism ...think of it as SN2-like.
Hope this helps.
Dr. Romano
- Joined
- Jun 9, 2014
- Messages
- 1,443
- Reaction score
- 771
If it makes it easier, picture it like a nucleophilic acyl sub. rxn....acyl chloride with alcohol forms ester....sulfonic ester formed like Dr. Romano stated previously.Oh so is this just like the O Ts reactions?
Similar threads
- Replies
- 1
- Views
- 1K