Maybe the better question is when does hydroxide act as a base vs. nucleophile? In a gem-diol rxn under basic conditions -OH acts as a :nu attacking carbonyl carbon. In an aldol condensation the -OH functions as a :nu and abstracts alpha H. I haven't had an aldol condensation question where I've also had the conditions (acidic vs basic). How do you predict how -OH behaves?
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
When does hydroxide abstract an alpha H vs. attacking carbonyl carbon?
- Thread starter d999
- Start date
D
deleted407021
SDN is not for homework help. Just read the book or ask your professor.
EDIT: Alrighty. My bad.
EDIT: Alrighty. My bad.
Last edited by a moderator:
- Joined
- Aug 13, 2008
- Messages
- 1,813
- Reaction score
- 1,145
it DOES attack as a nucleophile... but that leads to the geminal diol (which is NOT isolatable). In other words, the geminal diol is in equililbrium with the unmolested carbonyl. So we have
Geminal diol <---> Ketone/aldehyde (protonated at alpha position) <---> Enolate ion (deprotonated at alpha position) .
(Equilibrium between the ketone and the geminal diol is clearly driven to the right)
Geminal diol <---> Ketone/aldehyde (protonated at alpha position) <---> Enolate ion (deprotonated at alpha position) .
(Equilibrium between the ketone and the geminal diol is clearly driven to the right)
Last edited:
- Joined
- Sep 19, 2012
- Messages
- 5,149
- Reaction score
- 4,609
SDN is not for homework help. Just read the book or ask your professor.
Translate: I don't know the answer.
D
deleted407021
Translate: I don't know the answer.
lol nope. I haven't come across those rxns. Maybe they are going by a different name than what I learned?
- Joined
- Sep 19, 2012
- Messages
- 5,149
- Reaction score
- 4,609
I don't know it either 😳😛lol nope. I haven't come across those rxns. Maybe they are going by a different name than what I learned?
it DOES attack as a nucleophile... but that leads to the geminal diol (which is NOT isolatable). In other words, the geminal diol is in equililbrium with the unmolested carbonyl. So we have
Geminal diol <---> Ketone/aldehyde (protonated at alpha position) <---> Enolate ion (deprotonated at alpha position) .
(Equilibrium between the ketone and the geminal diol is clearly driven to the right)
Thanks very much. What should I look for when predicting how the -OH will behave with a ketone? The gem-diol and aldol condensation rxns were just examples. Like in hemiacetal rxns, the alcohol acts as a :nu (like the hydroxide in a gem-diol rxn) to attack a carbonyl carbon. How the heck am I supposed to predict how the OH group will act?
- Joined
- Jul 3, 2006
- Messages
- 792
- Reaction score
- 224
dear god the state of ochem in this country. OH does BOTH, silly. it's a thermodyanamic reaction -- in an aldol condensation reaction almost every step is reversible until the CONDENSATION step -- it's a thermodynamic sink. in an acetal protection reaction, deprotonation of the alpha carbon results in a non-productive step, and thus it reverses. closure of the acetal results in a thermodynamically stable product (in base, with a H2O trap), which is why the reaction proceeds to the right.Maybe the better question is when does hydroxide act as a base vs. nucleophile? In a gem-diol rxn under basic conditions -OH acts as a :nu attacking carbonyl carbon. In an aldol condensation the -OH functions as a :nu and abstracts alpha H. I haven't had an aldol condensation question where I've also had the conditions (acidic vs basic). How do you predict how -OH behaves?
- Joined
- Aug 13, 2008
- Messages
- 1,813
- Reaction score
- 1,145
Yes, alcohols will behave similar to water(hydroxide) .... however, you must be aware that when you have added 2 units of alcohol, you call the species an "ACETAL" (or "ketal"), which IS INDEED isolatable. So you COULD consider that to be a 'final product' (unlike the geminal diol)Thanks very much. What should I look for when predicting how the -OH will behave with a ketone? The gem-diol and aldol condensation rxns were just examples. Like in hemiacetal rxns, the alcohol acts as a :nu (like the hydroxide in a gem-diol rxn) to attack a carbonyl carbon. How the heck am I supposed to predict how the OH group will act?
- Joined
- Aug 13, 2008
- Messages
- 1,813
- Reaction score
- 1,145
Formation of the Carbon-carbon bond is reversible? I would think that would be the first irreversible step of any aldol reaction.dear god the state of ochem in this country. OH does BOTH, silly. it's a thermodyanamic reaction -- in an aldol condensation reaction almost every step is reversible until the CONDENSATION step -- it's a thermodynamic sink. in an acetal protection reaction, deprotonation of the alpha carbon results in a non-productive step, and thus it reverses. closure of the acetal results in a thermodynamically stable product (in base, with a H2O trap), which is why the reaction proceeds to the right.
- Joined
- Jul 3, 2006
- Messages
- 792
- Reaction score
- 224
indeed formation of a C-C can be reversible. There are such things as reverse/retro aldol reactions. it's just that most of the time elementary organic chemistry doesn't each this.Formation of the Carbon-carbon bond is reversible? I would think that would be the first irreversible step of any aldol reaction.
- Joined
- Aug 13, 2008
- Messages
- 1,813
- Reaction score
- 1,145
I believe you I just want to see one 😉 (I'm picturing an electrocyclic step that breaks a small(strained) ring)indeed formation of a C-C can be reversible. There are such things as reverse aldol reactions. it's just that most of the time elementary organic chemistry doesn't each this.
- Joined
- Jul 3, 2006
- Messages
- 792
- Reaction score
- 224
lol actually it's not that exciting. in a completely in vitro (organic) setting, it is actually quite a feat to pull off. In biology, retroaldols occur ALL THE TIME -- hello, fat hydrolysis.I believe you I just want to see one 😉 (I'm picturing an electrocyclic step that breaks a small(strained) ring)
- Joined
- Jul 3, 2006
- Messages
- 792
- Reaction score
- 224
i know. biology = magic!!!!True, the acetate pathway as well as the citric acid cycle come to mind! 2C units, baby. Aldolase rings a bell
- Joined
- Sep 12, 2011
- Messages
- 1,781
- Reaction score
- 2,203
I think the answer has to do with kinetics. Acid/base is much faster than nucleophilic attack at a carbonyl, not to mention, acid base rxn will have lower energy "transition state" compared to nucleophilic addition product.
- Joined
- Jul 3, 2006
- Messages
- 792
- Reaction score
- 224
lol well it's either kinetics or thermodynamics. because the two govern everything haha.I think the answer has to do with kinetics. Acid/base is much faster than nucleophilic attack at a carbonyl, not to mention, acid base rxn will have lower energy "transition state" compared to nucleophilic addition product.
- Joined
- Sep 12, 2011
- Messages
- 1,781
- Reaction score
- 2,203
indeed formation of a C-C can be reversible. There are such things as reverse/retro aldol reactions. it's just that most of the time elementary organic chemistry doesn't each this.
if intro org taught that, or More O'ferral jencks plots, or stereoselective synthesis pre-meds would riot.
- Joined
- Jul 3, 2006
- Messages
- 792
- Reaction score
- 224
haha they already riot. oh wells. 😀if intro org taught that, or More O'ferral jencks plots, or stereoselective synthesis pre-meds would riot.
- Joined
- Jul 24, 2010
- Messages
- 2,613
- Reaction score
- 740
Looks like I'm in the nerdy part of SDN again....
- Joined
- Jun 2, 2013
- Messages
- 624
- Reaction score
- 361
Maybe the better question is when does hydroxide act as a base vs. nucleophile? In a gem-diol rxn under basic conditions -OH acts as a :nu attacking carbonyl carbon. In an aldol condensation the -OH functions as a :nu and abstracts alpha H. I haven't had an aldol condensation question where I've also had the conditions (acidic vs basic). How do you predict how -OH behaves?
They are two competing reactions. Think about the pKa... with formaldehyde, H2-C=O, the hydrogen would be almost impossible to pull off because resonance isn't possible and an entire negative charged is placed on the carbonyl carbon. Therefore only carbonyl attack is possible and forming a gem idol.
In aldol condensation with acetaldehyde H3C-CH=O, the hydrogen on H3C is much easier to pull off because the delta positive carbonyl carbon acting as an electron sink (pretty much sharing a negative charge across two carbons) and the resonance stabilizing effect, with the extra electrons from hydrogen forming a double C=C bond and pushing the C=O double bond into C-O single bond.
H2C(-)-C(delta +)H=O <----> H2C=CH-O(-) *Parentheses indicating where the charges are located. This is a much more favorable structure and therefore, more likely that an aldol condensation will be possible compared to just formaldehyde.
P.S., on a side note, malonic ester synthesis uses similar principle to pull a hydrogen off of the beta carbon to start the reaction.
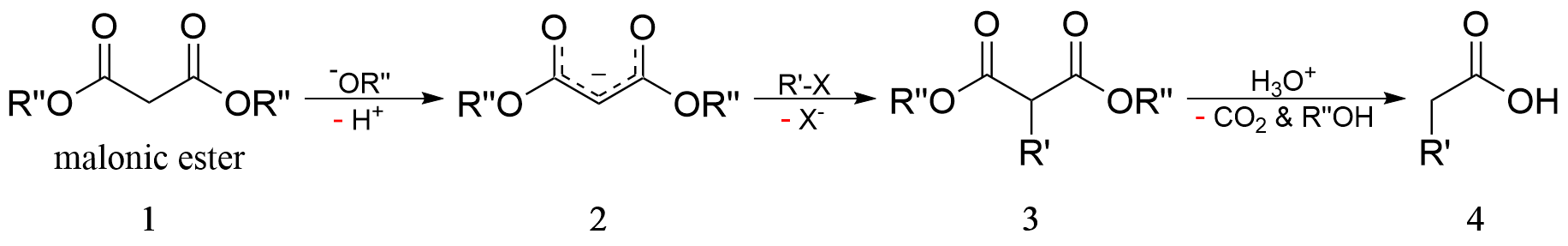
Last edited:
- Joined
- Jan 2, 2014
- Messages
- 11,384
- Reaction score
- 24,027
If you have basic conditions that means you have a lot of OH- groups floating around (it's not really OH- floating around in the solution, it's more like cages of H-O-H bonds with a few Hs left out but that's the easiest way to think of it). Then there's a greater chance of it attacking the carbonyl carbon.
Similar threads
- Replies
- 6
- Views
- 1K
- Replies
- 0
- Views
- 295
