- Joined
- Jul 22, 2011
- Messages
- 1,507
- Reaction score
- 318
- Points
- 5,171
- Pre-Medical
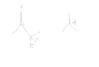
So, I know that electron withdrawing groups generally make compounds more acidic. But what if the central atom is partially positive, not negative in a way that withdrawing groups DESTABILIZE it instead of STABILIZING it? I attached a picture to help you understand what I am talking about.
In this case, is the right one more acidic as it is more stable?
The central C of the left one loses even more electrons because of inductive effect by F, so it becomes even more positive and becomes less stable..
In this case, is the right one more acidic as it is more stable?
The central C of the left one loses even more electrons because of inductive effect by F, so it becomes even more positive and becomes less stable..