- Joined
- Jun 23, 2013
- Messages
- 958
- Reaction score
- 413
While reviewing E2/E1 stuff, I came across something... I'm a little confused as to why DBN is considered a strong base. Usually when I rank the stability of the base qualitatively, I look to see if there's any stabilizing factors. In the case of DBN (below), I would think that because the lone pair exhibits resonance stabilization that it would be a weak base. But instead, this book I'm using for reference says otherwise. Apparently they're looking at the stability of what happens AFTER it abstracts a proton and the stability that results from the conjugate acid. I dunno, but doesn't that seem a little contradicting? I understand why it's conjugate acid is weak, but to even be protonated, you'd have to be a reasonable basic compound.
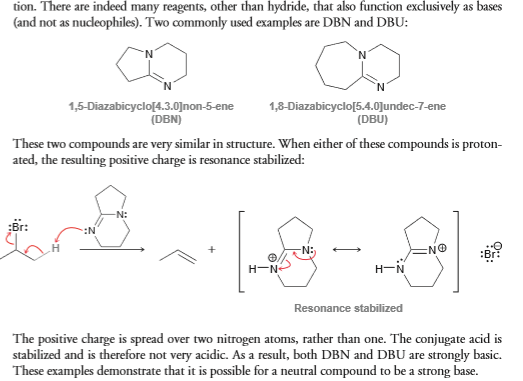
I mean don't most people rank base stability by looking at the conjugate base rather than the acid? I'm so confused now.
I mean don't most people rank base stability by looking at the conjugate base rather than the acid? I'm so confused now.
